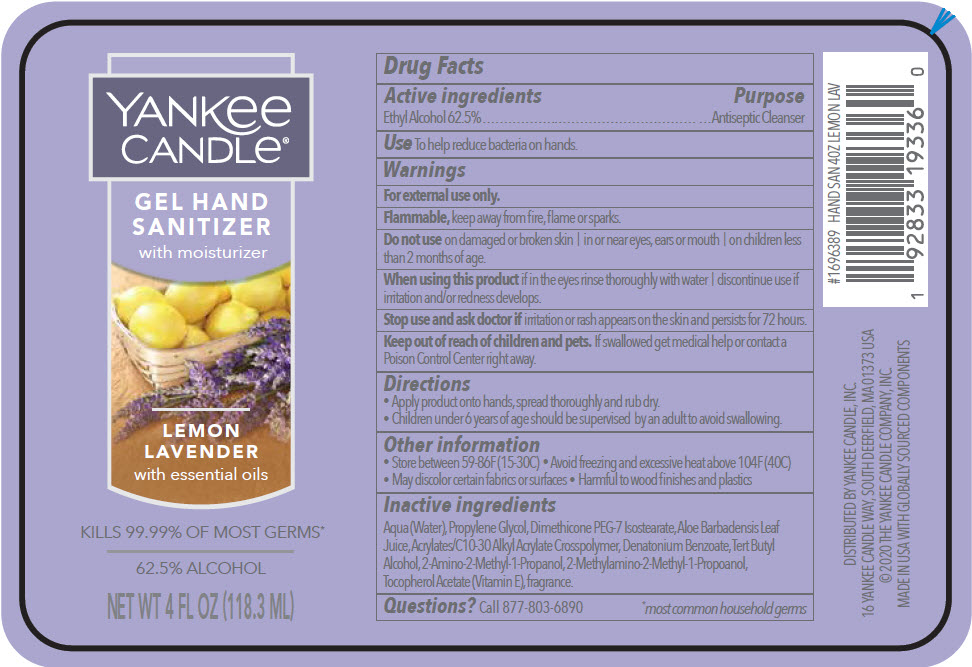
YANKEE CANDLE HAND SANITIZER WITH MOISTURIZER LEMON LAVENDER- alcohol gel
THE YANKEE CANDLE COMPANY INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Ethyl Alcohol 62.5%
Purpose
Antiseptic Cleanser
Use
To help reduce bacteria on hands.
Warnings
For external use only.
Flammable, keep away from fire, flame or sparks.
Do not use on damaged or broken skin | in or near eyes, ears or mouth | on children less than 2 months of age.
When using this product if in the eyes rinse thoroughly with water | discontinue use if irritation and/or redness develops.
Stop use and ask doctor if irritation or rash appears on the skin and persists for 72 hours.
Keep out of reach of children and pets. If swallowed get medical help or contact a Poison Control Center right away.
Directions
- Apply product onto hands, spread thoroughly and rub dry.
- Children under 6 years of age should be supervised by an adult to avoid swallowing.
Other information
- Store between 59-86F (15-30C)
- Avoid freezing and excessive heat above 104F (40C)
- May discolor certain fabrics or surfaces
- Harmful to wood finishes and plastics
Inactive ingredients
Aqua (Water), Propylene Glycol, Dimethicone PEG-7 Isostearate, Aloe Barbadensis Leaf Juice, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Denatonium Benzoate, Tert Butyl Alcohol, 2-Amino-2-Methyl-1-Propanol, 2-Methylamino-2-Methyl-1-Propoanol, Tocopherol Acetate (Vitamin E), fragrance.
Questions?
Call 877-803-6890
DISTRIBUTED BY YANKEE CANDLE, INC.
16 YANKEE CANDLE WAY, SOUTH DEERFIELD, MA 01373 USA
PRINCIPAL DISPLAY PANEL - 118.3 ML Bottle Label
YANKee
CANDLe®
GEL HAND
SANITIZER
with moisturizer
LEMON
LAVENDER
with essential oils
KILLS 99.99% OF MOST GERMS*
62.5% ALCOHOL
NET WT 4 FL OZ (118.3 ML)