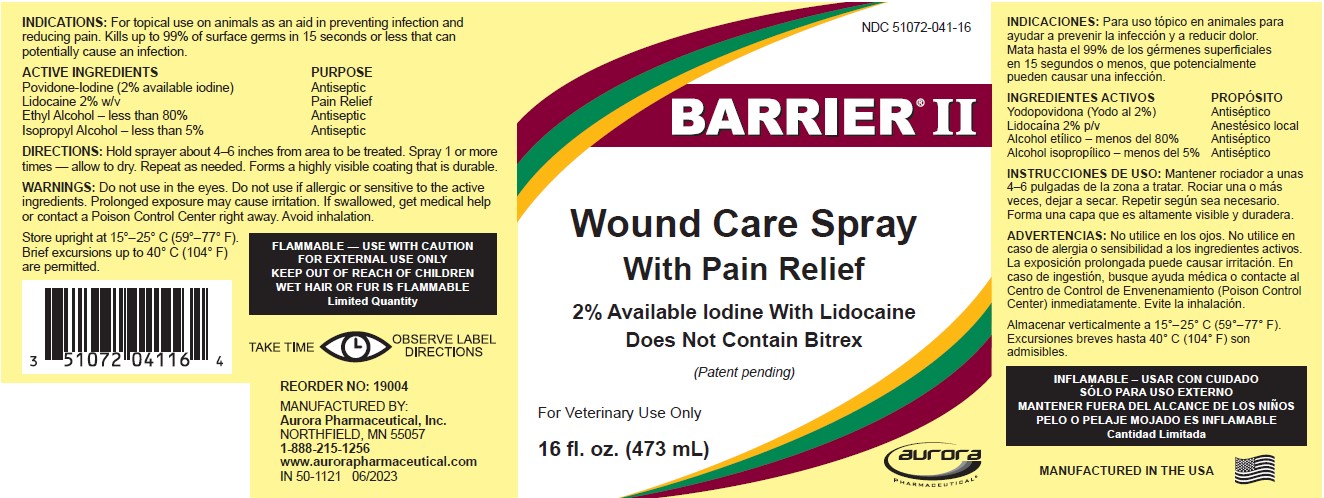
INDICATIONS
For topical use on animals as an aid in preventing infection and reducing pain. Kills up to 99% of surface germs in 15 seconds or less that can potentially cause an infection.
| ACTIVE INGREDIENTS | PURPOSE |
|---|---|
| Povidone-Iodine (2% available iodine) | Antiseptic |
| Lidocaine 2% w/v | Pain Relief |
| Ethyl Alcohol – less than 80% | Antiseptic |
| Isopropyl Alcohol – less than 5% | Antiseptic |
DIRECTIONS
Hold sprayer about 4-6 inches from area to be treated. Spray 1 or more times - allow to dry. Repeat as needed. Forms a highly visible coating that is durable.
WARNINGS
Do not use in the eyes. Do not use if allergic or sensitive to the active ingredients. Prolonged exposure may cause irritation. If swallowed, get medical help or contact a Poison Control Center right away. Avoid inhalation.
FLAMMABLE — USE WITH CAUTION
FOR EXTERNAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
WET HAIR OR FUR IS FLAMMABLE
Limited Quantity