Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
Directions
- •
- do not crush, chew, or break tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for the timing of meals
- •
- adults and children 12 years of age and over: 1 tablet every 12 hours. Do not exceed 2 tablets in 24 hours.
- •
- children under 12 years of age: do not use
Other information
- •
- Tamper evident: Do not use if carton is open or if printed seal on blister is broken or missing.
- •
- store between 20-25°C (68-77°F)
Inactive ingredients
carbomer homopolymer type B; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
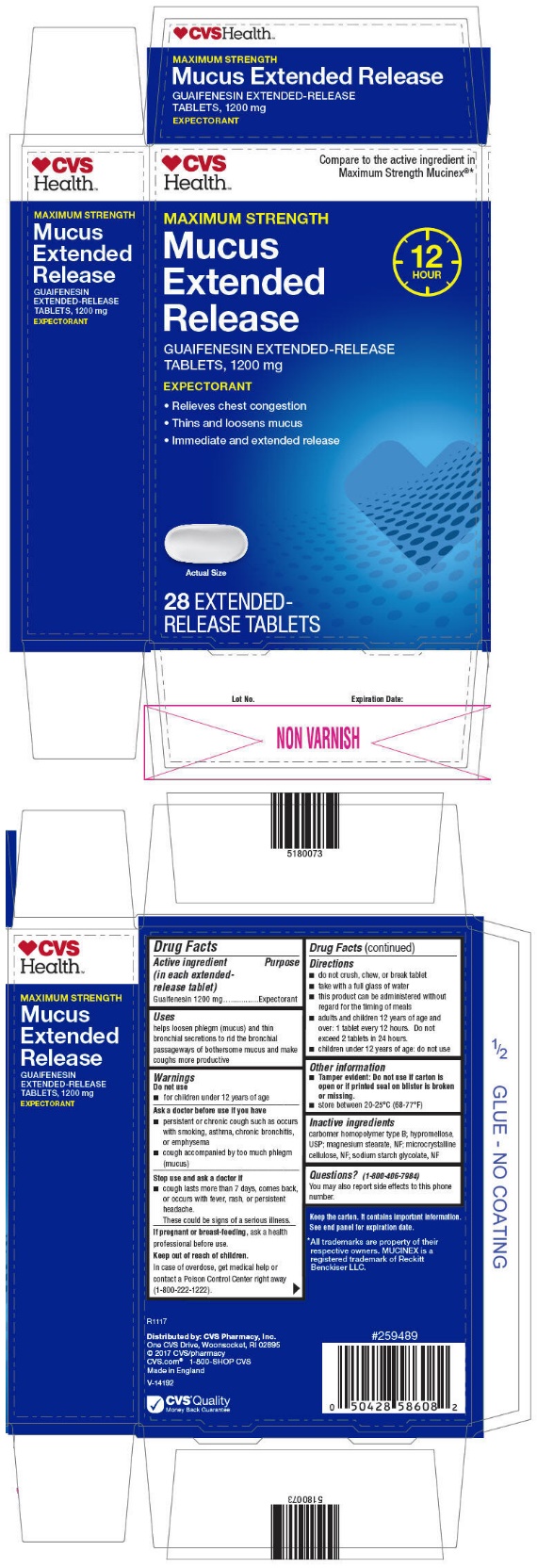
PRINCIPAL DISPLAY PANEL - 1200 mg Tablet Blister Pack Carton
CVS
Health™
Compare to the active ingredient in
Maximum Strength Mucinex®*
MAXIMUM STRENGTH
Mucus
Extended
Release
12
HOUR
GUAIFENESIN EXTENDED-RELEASE
TABLETS, 1200 mg
EXPECTORANT
- •
- Relieves chest congestion
- •
- Thins and loosens mucus
- •
- Immediate and extended release
Actual Size
28 EXTENDED-
RELEASE TABLETS