ATRIDOX- doxycycline hyclate
TOLMAR Inc.
----------
ATRIDOX®
(doxycycline hyclate) 10%
in the ATRIGEL® Delivery System
for controlled release in
subgingival application
DESCRIPTION
The ATRIDOX® product* is a subgingival controlled-release product composed of a two syringe mixing system. Syringe A contains 450 mg of the ATRIGEL® Delivery System, which is a bioabsorbable, flowable polymeric formulation composed of 36.7% poly(DL-lactide) (PLA) dissolved in 63.3% N-methyl-2-pyrrolidone (NMP). Syringe B contains 50 mg of doxycycline hyclate which is equivalent to 42.5 mg doxycycline. The constituted product is a pale yellow to yellow viscous liquid with a concentration of 10% of doxycycline hyclate. Upon contact with the crevicular fluid, the liquid product solidifies and then allows for controlled release of drug for a period of 7 days.
*ATRIDOX® is a registered trademark of TOLMAR Inc. ATRIGEL® is a registered trademark of TOLMAR Therapeutics, Inc.
Doxycycline is a broad-spectrum antibiotic synthetically derived from oxytetracycline.
The structural formula of doxycycline hyclate is:
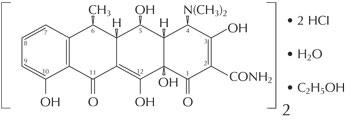
Empirical Formula: (C22H24N2O8•HCI)2•C2H6O•H2O
CLINICAL PHARMACOLOGY
Microbiology
Doxycycline is a broad-spectrum semisynthetic tetracycline.1 Doxycycline is bacteriostatic, inhibiting bacterial protein synthesis due to disruption of transfer RNA and messenger RNA at ribosomal sites.1In vitro testing has shown that Porphyromonas gingivalis, Prevotella intermedia, Campylobacter rectus, and Fusobacterium nucleatum, which are associated with periodontal disease, are susceptible to doxycycline at concentrations ≤ 6.0 µg/mL.2 A single-center, single-blind, randomized, clinical study in 45 subjects with periodontal disease demonstrated that a single treatment with ATRIDOX® resulted in the reduction in the numbers of P. gingivalis, P. intermedia, C. rectus, F. nucleatum, Bacteroides forsythus, and E. corrodens in subgingival plaque samples. Levels of aerobic and anaerobic bacteria were also reduced after treatment with ATRIDOX®. The clinical significance of these findings, however, is not known. During these studies, no overgrowth of opportunistic organisms such as Gram-negative bacilli and yeast were observed. However, as with other antibiotic preparations, ATRIDOX® therapy may result in the overgrowth of nonsusceptible organisms including fungi. (See PRECAUTIONS)
Pharmacokinetics
In a clinical pharmacokinetic study, subjects were randomized to receive either ATRIDOX® covered with Coe-Pak™ periodontal dressing (n=13), ATRIDOX® covered with Octyldent™ periodontal adhesive (n=13), or oral doxycycline (n=5) (according to package dosing instructions). The doxycycline release characteristics in gingival crevicular fluid (GCF), saliva, and serum were evaluated.
Doxycycline levels in GCF peaked (~1,500 µg/mL and ~2000 µg/mL for Coe-Pak™ and Octyldent™ groups, respectively) 2 hours following treatment with ATRIDOX®. These levels remained above 1000 µg/mL through 18 hours, at which time the levels began to decline gradually. However, local levels of doxycycline remained well above the minimum inhibitory concentration (MIC90) for periodontal pathogens (≤ 6.0 µg/mL)2 through Day 7. In contrast, subjects receiving oral doxycycline had peak GCF levels of ~2.5 µg/mL at 12 hours following the initial oral dosing with levels declining to ~0.2 µg/mL by Day 7. High variability was observed for doxycycline levels in GCF for both oral and ATRIDOX® treatment groups.
The ATRIDOX® doxycycline release profile in GCF is illustrated in the figure below.
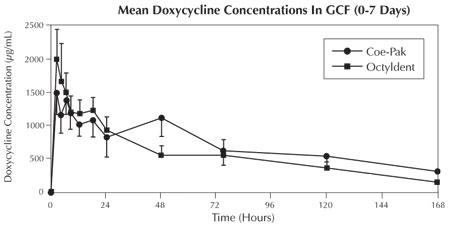
The maximum concentration of doxycycline in saliva was achieved at 2 hours after both treatments with ATRIDOX®, with means of 4.05 µg/mL and 8.78 µg/mL and decreased to 0.36 µg/mL and 0.23 µg/mL at Day 7 for the Coe-Pak™ group and the Octyldent™ group, respectively.
The concentration of doxycycline in serum following treatment of ATRIDOX® never exceeded 0.1 µg/mL.
CLINICAL STUDIES
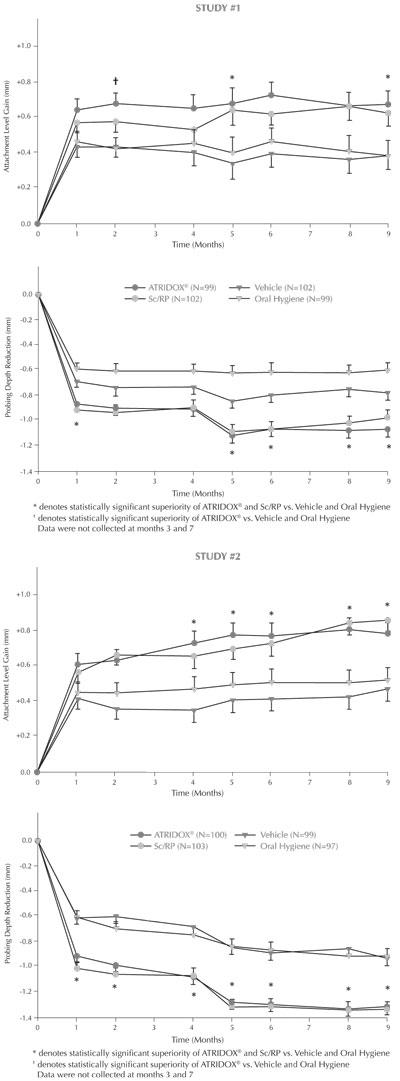
In two well-controlled, multicenter, parallel-design, nine-month clinical trials, 831 patients (Study 1=411; Study 2=420) with chronic adult periodontitis characterized by a mean probing depth of 5.9 to 6.0 mm were enrolled. Subjects received one of four treatments: 1) ATRIDOX®, 2) Scaling and Root Planing, 3) Vehicle Control, or 4) Oral Hygiene. Treatment was administered to sites with probing depths 5 mm or greater that bled on probing. Subjects with detectable subgingival calculus on greater than 80% of all tooth surfaces were excluded from enrollment. All subjects received a second administration of the initially randomized treatment four months after their Baseline treatment. Changes in the efficacy parameters, attachment level, pocket depth, and bleeding on probing, between Baseline and Month 9 showed that: 1) ATRIDOX® was superior to Vehicle Control and Oral Hygiene, and 2) ATRIDOX® met the decision rule of being at least 75% as good as Scaling and Root Planing (SRP) (the standard of at least 75% as good as SRP is required for any product approved as a stand alone therapy for periodontitis). Clinicians should note that the studies were of nine months duration. Additional research would be necessary to establish long term comparability to SRP. The results of Studies #1 and 2 for efficacy parameters of attachment level gain and probing depth reduction are included in the following graphs.
A third clinical trial was conducted to determine whether the product can be left in the pocket to bioabsorb or be expelled naturally and achieve comparable clinical results. In this study the product was retained with Octyldent™ dental adhesive rather than Coe-Pak™ periodontal dressing as in the previously mentioned studies. This was a 3-arm, randomized, controlled, parallel group, single blind trial that enrolled 605 subjects. The patient population studied and study design were comparable to that in Studies 1 and 2. Subjects received one of three treatments: 1) ATRIDOX® with Coe-Pak™ removed after 7 days as in the pivotal trials, 2) ATRIDOX® retained with Octyldent™ and left to bioabsorb or be expelled naturally or 3) Vehicle Control with Octyldent™ left to bioabsorb or be expelled naturally. Changes in the efficacy parameters, attachment level, pocket depth and bleeding on probing were equivalent to those observed in Studies 1 and 2. The results of the third study support the use of ATRIDOX® retained with Octyldent™ and left to bioabsorb or be expelled naturally.
INDICATIONS AND USAGE
ATRIDOX® is indicated for use in the treatment of chronic adult periodontitis for a gain in clinical attachment, reduction in probing depth, and reduction in bleeding on probing.
CONTRAINDICATIONS
ATRIDOX® should not be used in patients who are hypersensitive to doxycycline or any other drug in the tetracycline class.
WARNINGS
THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY, AND CHILDHOOD TO THE AGE OF EIGHT YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH. This adverse reaction is more common during long-term use of the drugs, but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. TETRACYCLINE DRUGS, THEREFORE, SHOULD NOT BE USED IN THIS AGE GROUP, OR IN PREGNANT WOMEN, UNLESS OTHER DRUGS ARE NOT LIKELY TO BE EFFECTIVE OR ARE CONTRAINDICATED. Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can have toxic effects on the developing fetus (often related to skeletal development). Evidence of embryotoxicity has also been noted in animals treated early in pregnancy. If any tetracycline is used during pregnancy, the patient should be apprised of the potential hazard to the fetus.
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking doxycycline or other tetracyclines. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs.
PRECAUTIONS
General
ATRIDOX® has not been clinically tested in pregnant women.
ATRIDOX® has not been clinically evaluated in patients with conditions involving extremely severe periodontal defects with very little remaining periodontium.
ATRIDOX® has not been clinically tested for use in the regeneration of alveolar bone, either in preparation for or in conjunction with the placement of endosseous (dental) implants or in the treatment of failing implants.
ATRIDOX® has not been clinically tested in immunocompromised patients (such as patients immunocompromised by diabetes, chemotherapy, radiation therapy, or infection with HIV).
As with other antibiotic preparations, ATRIDOX® therapy may result in overgrowth of nonsusceptible organisms, including fungi.1 The effects of prolonged treatment, greater than six months, have not been studied.
ATRIDOX® should be used with caution in patients with a history of or predisposition to oral candidiasis. The safety and effectiveness of ATRIDOX® have not been established for the treatment of periodontitis in patients with coexistent oral candidiasis.
Information for Patients
Mechanical oral hygiene procedures (i.e., tooth brushing, flossing) should be avoided on any treated areas for 7 days.
Avoid excessive sunlight or artificial ultraviolet light while receiving doxycycline.
Doxycycline may decrease the effectiveness of birth control pills.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential of doxycycline have not been conducted. However, there has been evidence of oncogenic activity in rats in studies with the related antibiotics, oxytetracycline (adrenal and pituitary tumors), and minocycline (thyroid tumors). Likewise, although mutagenicity studies of doxycycline have not been conducted, positive results in in vitro mammalian cell assays have been reported for related antibiotics (tetracycline, oxytetracycline). Doxycycline administered orally at dosage levels as high as 250 mg/kg/day had no apparent effect on the fertility of female rats. Effect on male fertility has not been studied.
Pregnancy Category D. See “WARNINGS” section.
Nursing Mothers
Tetracyclines appear in breast milk following oral administration. It is not known whether doxycycline is excreted in human milk following use of ATRIDOX®. Because of the potential for serious adverse reactions in nursing infants from doxycycline, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. (See WARNINGS)
ADVERSE REACTIONS
In clinical trials involving a total of 1436 patients, adverse experiences from all causalities were monitored across treatment groups.
In the Circulatory System category, 10 subjects (1.6%) in the ATRIDOX® group were reported as having "unspecified essential hypertension." Only 1 subject (0.2%) in the Vehicle group, and none in the Scaling and Root Planing or Oral Hygiene groups were reported to have "unspecified essential hypertension." In all cases, the event occurred anywhere from 13 to 134 days post treatment. There is no known association of oral administration of doxycycline with essential hypertension.
Two patients in the polymer vehicle group and none in the ATRIDOX® group (0.2% for both groups combined) reported adverse events consistent with a localized allergic response.
Sex, age, race and smoking status did not appear to be correlated with adverse events.
The following table lists the incidence of treatment-emergent adverse events from all causalities, across all treatment groups, occurring in ≥1% of the entire study population.
| Body System | Doxycycline | Vehicle | OH | SRP |
| Verbatim Terms | n=609 | n=413 | n=204 | n=210 |
| Circulatory | ||||
| High blood pressure | 1.6% | 0.2% | 0.0% | 0.0% |
| Digestive | ||||
| Gum discomfort, pain or soreness; loss of attachment; increased pocket depth | 18.1% | 23.0% | 20.1% | 21.0% |
| Toothache, pressure sensitivity | 14.3% | 14.3% | 10.3% | 18.1% |
| Periodontal abscess, exudate, infection, drainage, extreme mobility, suppuration | 9.9% | 10.9% | 10.3% | 8.6% |
| Thermal tooth sensitivity | 7.7% | 8.5% | 4.4% | 6.7% |
| Gum inflammation, swelling, sensitivity | 4.1% | 5.8% | 5.4% | 5.7% |
| Soft tissue erythema, sore mouth, unspecified pain | 4.3% | 5.3% | 2.7% | 6.2% |
| Indigestion, upset stomach, stomachache | 3.6% | 4.1% | 2.9% | 3.8% |
| Diarrhea | 3.3% | 2.4% | 1.0% | 1.0% |
| Tooth mobility, bone loss | 2.0% | 0.7% | 0.5% | 2.4% |
| Periapical abscess, lesion | 1.5% | 1.9% | 1.0% | 0.5% |
| Aphthous ulcer, canker sores | 0.7% | 1.7% | 1.0% | 1.4% |
| Fistula | 0.8% | 1.5% | 1.5% | 1.0% |
| Endodontic abscess, pulpitis | 1.5% | 1.5% | 0.0% | 0.5% |
| Jaw pain | 1.1% | 0.5% | 1.0% | 1.9% |
| Tooth loss | 0.8% | 1.5% | 1.5% | 0.0% |
| Bleeding gums | 1.0% | 0.7% | 0.0% | 2.4% |
| Genitourinary | ||||
| Premenstrual tension syndrome | 4.4% | 3.1% | 2.5% | 3.3% |
| Ill-Defined Conditions | ||||
| Headache | 27.3% | 28.1% | 23.5% | 23.8% |
| Cough | 3.6% | 6.1% | 2.9% | 2.4% |
| Sleeplessness | 3.4% | 1.5% | 2.0% | 2.9% |
| Body aches, soreness | 1.6% | 1.2% | 1.5% | 1.4% |
| Nausea and vomiting | 1.8% | 0.7% | 2.5% | 0.5% |
| Fever | 1.0% | 1.9% | 1.0% | 1.9% |
| Injury & Poisoning | ||||
| Broken tooth | 5.1% | 4.1% | 4.9% | 5.7% |
| Mental | ||||
| Tension headache | 1.8% | 0.7% | 0.0% | 1.0% |
| Musculoskeletal | ||||
| Muscle aches | 6.4% | 4.6% | 4.9% | 3.3% |
| Backache | 3.6% | 5.3% | 2.5% | 6.2% |
| Pain in arms or legs | 1.5% | 2.2% | 2.0% | 2.4% |
| Lower back pain | 1.6% | 1.7% | 0.5% | 2.9% |
| Neck pain | 1.3% | 1.7% | 1.0% | 1.9% |
| Shoulder pain | 1.0% | 1.0% | 1.5% | 1.0% |
| Nervous System | ||||
| Ear infection | 1.6% | 1.9% | 2.0% | 0.0% |
| Respiratory | ||||
| Common cold | 25.5% | 25.2% | 18.1% | 16.7% |
| Flu, respiratory | 6.1% | 9.0% | 3.9% | 6.7% |
| Stuffy head, post nasal drip, congestion | 5.6% | 7.7% | 2.9% | 4.8% |
| Sore throat | 5.7% | 6.5% | 2.0% | 3.3% |
| Sinus infection | 5.3% | 2.7% | 1.0% | 1.9% |
| Flu | 2.8% | 2.9% | 2.9% | 3.3% |
| Bronchitis | 2.3% | 1.9% | 1.5% | 1.0% |
| Allergies | 1.0% | 1.0% | 1.0% | 1.9% |
| Skin & Subcutaneous Tissue | ||||
| Skin infection or inflammation | 1.3% | 1.0% | 1.0% | 1.0% |
DOSAGE AND ADMINISTRATION
ATRIDOX® is a variable dose product dependent on the size, shape, and number of pockets being treated.
Preparation for Use
- If refrigerated, remove the product from refrigeration at least 15 minutes prior to mixing.
- Couple Syringe A (liquid delivery system) and Syringe B (drug powder).
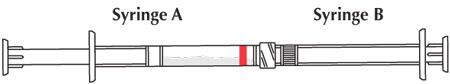
- Inject the liquid contents of Syringe A (indicated by red stripe) into Syringe B (doxycycline powder) and then push the contents back into Syringe A. This entire operation is one mixing cycle.
- Complete 100 mixing cycles at a pace of one cycle per second using brisk strokes.
If immediate use is desired, skip to step 7.
-
If necessary, the coupled syringes can be stored at room temperature for a maximum of three days. Some of the ATRIDOX® systems are packaged in resealable pouches that can be used for this purpose. If the ATRIDOX® system is packaged in a tray, use an airtight container.
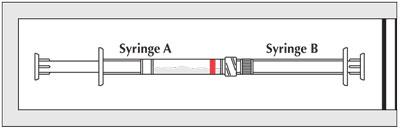
- After storage, perform an additional ten mixing cycles just prior to use.
Continue with immediate use instructions.
- The contents will be in Syringe A (indicated by red stripe). Hold the coupled syringes vertically with Syringe A at the bottom. Pull back on the Syringe A plunger and allow the contents to flow down the barrel for several seconds.
- Uncouple the two syringes and attach one of the provided cannulae to Syringe A.
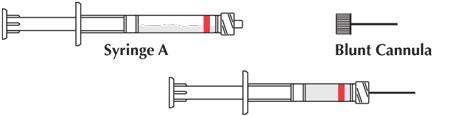
Product is now ready for application.
Product Administration
ATRIDOX® does not require local anesthesia for placement. Bend the cannula to resemble a periodontal probe and explore the periodontal pocket in a manner similar to periodontal probing. Keeping the cannula tip near the base of the pocket, express the product into the pocket until the formulation reaches the top of the gingival margin. Withdraw the cannula tip from the pocket. In order to separate the tip from the formulation, turn the tip of the cannula towards the tooth, press the tip against the tooth surface, and pinch the string of formulation from the tip of the cannula. Variations on this technique may be needed to achieve separation between ATRIDOX® and cannula.
If desired, using an appropriate dental instrument, ATRIDOX® may be packed into the pocket. Dipping the edge of the instrument in water before packing will help keep ATRIDOX® from sticking to the instrument, and will help speed coagulation of ATRIDOX®. A few drops of water dripped onto the surface of ATRIDOX® once in the pocket will also aid in coagulation. If necessary, add more ATRIDOX® as described above and pack it into the pocket until the pocket is full.
Cover the pockets containing ATRIDOX® with either Coe-Pak™ periodontal dressing or a cyanoacrylate dental adhesive.
Application of ATRIDOX® may be repeated four months after initial treatment.
HOW SUPPLIED
The final blended product is 500 mg of formulation containing 50 mg of doxycycline hyclate (doxycycline hyclate, 10%).
ATRIDOX® is available as a tray or pouch containing a doxycycline hyclate syringe (50 mg), an ATRIGEL® Delivery System syringe (450 mg), and a blunt cannula. The pouched product is available in a box of six (NDC 63646-191-00), or box of two (NDC 63646-191-02), or a professional sample pouch (NDC 63646-191-01). The trayed product is available in a box of six (NDC 63646-191-05), a box of four (NDC 63646-191-04), or a professional sample box of two (NDC 63646-191-03).
Each ATRIDOX® syringe system is intended for use in only one patient. Do not use if packaging has been previously opened or damaged.
REFERENCES
1. Stratton CW, Lorian V. Mechanisms of action for antimicrobial agents: general principles and mechanisms for selected classes of
antibiotics. Antibiotics in Laboratory Medicine, 4th edition, Williams and Wilkins, Baltimore, MD, 1996.
2. Slots J, Rams TE. Antibiotics in periodontal therapy: advantages and disadvantages. J Clin Periodontol 1990; 17:479-493.
Manufactured by TOLMAR Inc.
Fort Collins, CO 80526
Distributed by Den-Mat Holdings, LLC
1017 W. Central Ave., Lompoc, CA 93436
Part Number: 44406 Rev. 6 11/14
ATRIDOX®
(doxycycline hyclate) 10%
in the ATRIGEL® Delivery System
for controlled release in
subgingival application
denmat
To Order Call: 1-800-433-6628
www.denmat.com
| ATRIDOX
doxycycline hyclate kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - TOLMAR Inc. (791156578) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TOLMAR Inc. | 791156578 | ANALYSIS(63646-191, 63646-193, 63646-192) , LABEL(63646-191, 63646-193, 63646-192) , MANUFACTURE(63646-191, 63646-193, 63646-192) , PACK(63646-191, 63646-193, 63646-192) | |