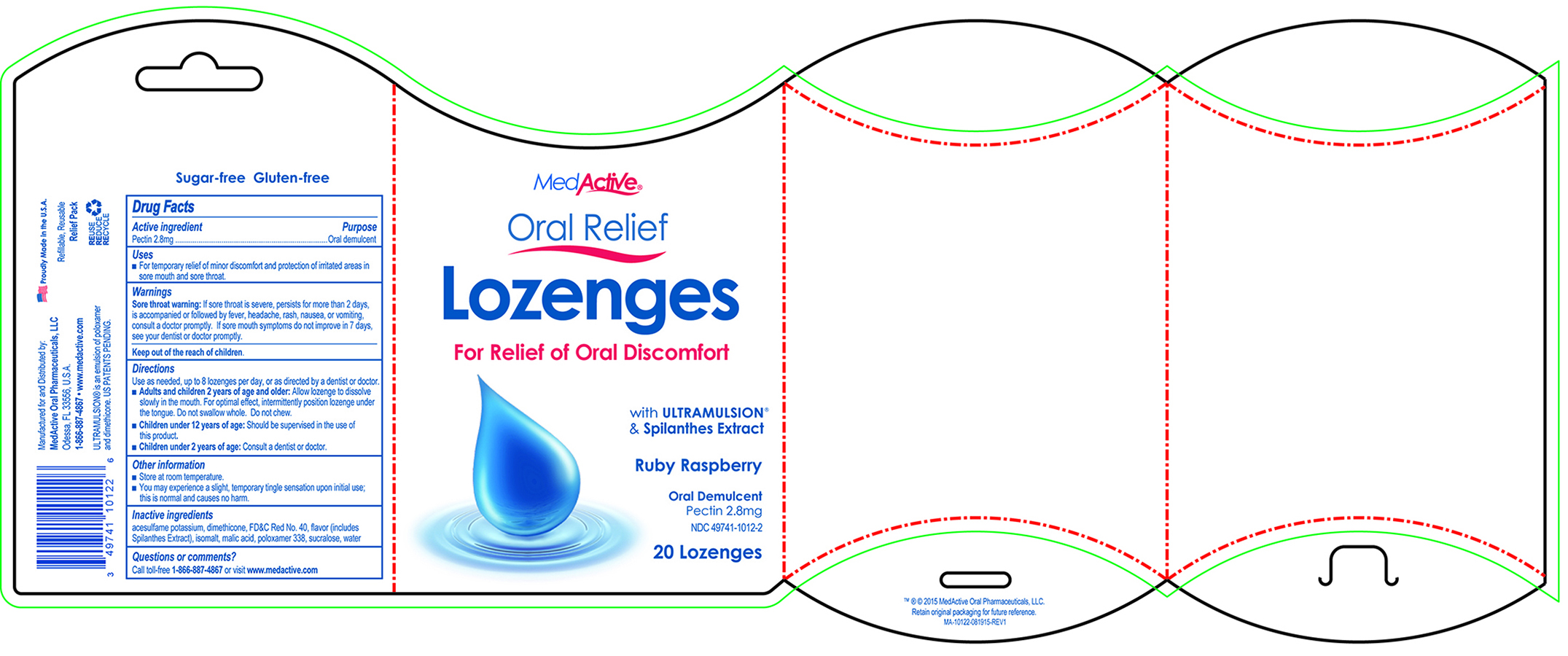
Uses
- For temporary relief of minor discomfort and protection of irritated areas in sore mouth and sore throat.
Sore Throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly. If sore mouth symptons do not improve in 7 days, see your dentist or doctor promptly.
Directions
Use as needed, up to 8 lozenges per day, or as directed by a dentist or doctor.
- Adults and children 2 years of age and older: Allow lozenge to dissolve slowly in the mouth. For optimal effect, intermittently position lozenge under the tongue. Do not swallow whole. Do not chew.
- Children under 12 years or age: Should be supervised in the use of this product.
- Children under 2 years of age: Consult a dentist or doctor.
Other Information
- Store at room temperature.
- You may experience a slight, temporary tingle sensation upon initial use; this is normal and causes no harm.