EPSOLAY- benzoyl peroxide cream
Sol-Gel Technologies Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EPSOLAY safely and effectively. See full prescribing information for EPSOLAY.
EPSOLAY® (benzoyl peroxide) cream, for topical use Initial U.S. Approval: 1984 INDICATIONS AND USAGEEPSOLAY is indicated for the treatment of inflammatory lesions of rosacea in adults. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSCream, 5%. (3) CONTRAINDICATIONSA history of a serious hypersensitivity reactions to benzoyl peroxide or any component of the formulation in EPSOLAY. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Sol-Gel Technologies Inc. at [1-866-SGT-AERS (748-4377)] or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2022 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
EPSOLAY is indicated for the treatment of inflammatory lesions of rosacea in adults.
2 DOSAGE AND ADMINISTRATION
- Before initial use, prime the pump until the first drop of cream is released.
- Apply a pea-sized amount of EPSOLAY once daily in a thin layer to each area of the face (forehead, chin, nose, each cheek) on clean and dry skin. Avoid the eyes, lips and mouth.
- Wash hands after application.
- EPSOLAY may bleach hair or colored fabric.
- EPSOLAY is for topical use only. Not for oral, ophthalmic, or intravaginal use.
- Discard unused EPSOLAY 30 days after first use.
3 DOSAGE FORMS AND STRENGTHS
Cream, 5%. Each gram of EPSOLAY contains 50 mg of benzoyl peroxide in a white to off-white base.
4 CONTRAINDICATIONS
EPSOLAY is contraindicated in patients with a history of hypersensitivity reactions to benzoyl peroxide or any components of the formulation in EPSOLAY [see Warnings and Precautions (5.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Hypersensitivity reactions, including anaphylaxis, angioedema, and urticaria, have been reported with the use of benzoyl peroxide products. If a serious hypersensitivity reaction occurs, discontinue EPSOLAY immediately and initiate appropriate therapy.
5.2 Skin Irritation/Contact Dermatitis
Erythema, scaling, dryness and stinging/burning may be experienced with use of EPSOLAY. Irritation and contact dermatitis may occur. Apply a moisturizer and discontinue EPSOLAY if symptoms do not improve. Avoid application of EPSOLAY to cuts, abrasions, eczematous or sunburned skin.
5.3 Photosensitivity
Benzoyl peroxide may increase sensitivity to sunlight. Minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using EPSOLAY. Instruct the patient to implement sun protection measures (e.g., sunscreen and loose-fitting clothes) when sun exposure cannot be avoided. Discontinue EPSOLAY at the first evidence of sunburn.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two randomized, double-blind, vehicle-controlled trials, adult subjects with rosacea applied EPSOLAY (N = 488) or vehicle (N = 234) once daily for 12 weeks. The majority of subjects were Caucasian (93%) and female (73%) with a mean age of 51 years.
Table 1 presents the most common adverse reactions occurring in ≥ 1% of subjects treated with EPSOLAY and more frequently than in subjects treated with vehicle.
| EPSOLAY N=488 | Vehicle N=234 |
|
|---|---|---|
|
||
| Application site pain | 11 (2%) | 2 (1%) |
| Application site erythema | 11 (2%) | 2 (1%) |
| Application site pruritus | 6 (1%) | 1 (<1%) |
| Application site edema* | 4 (1%) | 0 (0%) |
During the clinical trials, local tolerability evaluations were conducted at baseline and at each study visit by assessment of dryness, itching, scaling and stinging/burning. Table 2 presents the local tolerability assessments by severity grade at Week 12.
| Sign/Symptom | EPSOLAY N=455* |
||
|---|---|---|---|
| Severity at Week 12 | |||
| Mild | Moderate | Severe | |
|
|||
| Dryness | 25% | 7% | 0% |
| Itching | 24% | 6% | 0% |
| Scaling | 13% | 4% | 0% |
| Stinging/ Burning | 20% | 3% | 1% |
In a 40-week open-label extension safety study (for a total of up to 52 weeks of treatment) the frequency and severity of local tolerability signs and symptoms at Week 52 were comparable to those reported at Week 12.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The systemic exposure of benzoyl peroxide is unknown. Based on the published literature, benzoyl peroxide is metabolized to benzoic acid (an endogenous substance), which is eliminated in the urine. Hence, maternal use is not expected to result in fetal exposure to the drug. Animal reproductive studies have not been conducted with EPSOLAY or benzoyl peroxide.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of benzoyl peroxide in human milk, its effects on the breastfed infant or its effects on milk production. The systemic exposure of benzoyl peroxide is unknown. Based on the published literature, benzoyl peroxide is metabolized to benzoic acid (an endogenous substance), which is eliminated in the urine. Any amount of benzoyl peroxide excreted into human milk by a nursing mother would be expected to be metabolized by tissue and stomach esterases. Therefore, breastfeeding is not expected to result in exposure of the infant to EPSOLAY. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EPSOLAY and any potential adverse effects on the breastfed child from EPSOLAY or from the underlying maternal condition.
11 DESCRIPTION
EPSOLAY (benzoyl peroxide) cream is for topical use. Each gram of EPSOLAY contains 50 mg of benzoyl peroxide.
The chemical name for benzoyl peroxide is benzoyl benzenecarboperoxoate. It has the following structural formula:
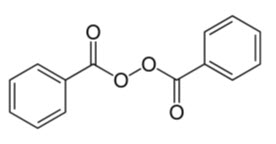
Molecular Formula: C14H10O4 Molecular Weight: 242.23
The benzoyl peroxide in EPSOLAY is in a solid form that is incorporated into a microcapsule composed of silicon dioxide, cetrimonium chloride and polyquaternium-7.
EPSOLAY contains anhydrous citric acid, cetrimonium chloride, cetyl alcohol, cyclomethicone, edetate disodium, glycerin, hydrochloric acid, lactic acid, macrogol stearate Type I, mono and di-glycerides, phenoxyethanol, polyquaternium-7, purified water, silicon dioxide and sodium hydroxide as inactive ingredients.
12 CLINICAL PHARMACOLOGY
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, mutagenicity, and impairment of fertility studies were not conducted with EPSOLAY.
The role of benzoyl peroxide as a tumor promoter has been well established in several animal species. However, the significance of this finding in humans is unknown.
No significant increase in tumor formation was observed in rats treated topically with a 15 to 25% benzoyl peroxide carbopol gel (3 to 5 times the concentration of benzoyl peroxide in EPSOLAY) for two years. Similar results were obtained in mice topically treated with 25% benzoyl peroxide gel for 56 weeks followed by intermittent treatment with 15% benzoyl peroxide gel for rest of the 2 years study period and in mice topically treated with 5% benzoyl peroxide gel for two years.
Bacterial mutagenicity assays (Ames test) conducted with benzoyl peroxide have provided mixed results; mutagenic potential was observed in a few studies but not in a majority of investigations. Benzoyl peroxide has been found to cause DNA strand breaks in a variety of mammalian cell types and to cause sister chromatid exchanges in Chinese hamster ovary cells.
Fertility studies were not conducted with benzoyl peroxide.
14 CLINICAL STUDIES
The safety and efficacy of EPSOLAY was evaluated in two multicenter, randomized, double-blind, vehicle-controlled trials (Trial 1 [NCT03448939] and Trial 2 [NCT03564119]) in subjects with moderate-to-severe papulopustular rosacea. The trials were conducted in 733 subjects, aged 18 years and older. Subjects were treated once daily for 12 weeks with either EPSOLAY or vehicle cream.
Subjects were required to have a minimum of 15 to 70 total inflammatory lesions (papules and/or pustules) and no more than 2 nodules (where a nodule was defined as a papule or pustule greater than 5 mm in diameter) and an Investigator Global Assessment (IGA) score of 3 ("moderate") or 4 ("severe") at baseline. Overall, 93% of subjects were Caucasian, 73% were female, and the mean age was 51 years (ranged from 18 to 85 years). At baseline, subjects had a mean inflammatory lesion count of 27.5, 89% were scored as moderate (IGA=3), and 11% scored as severe (IGA=4).
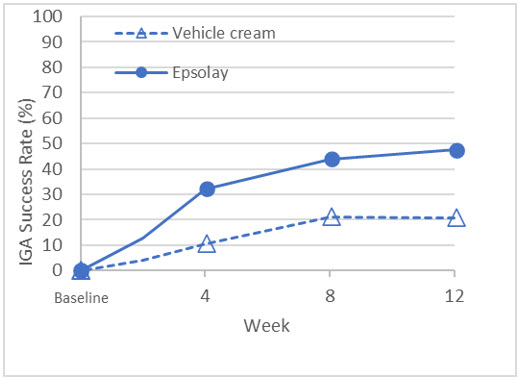
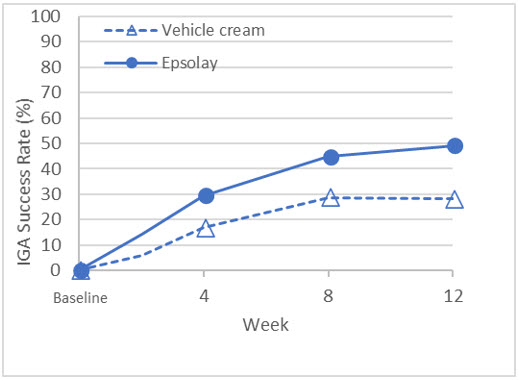
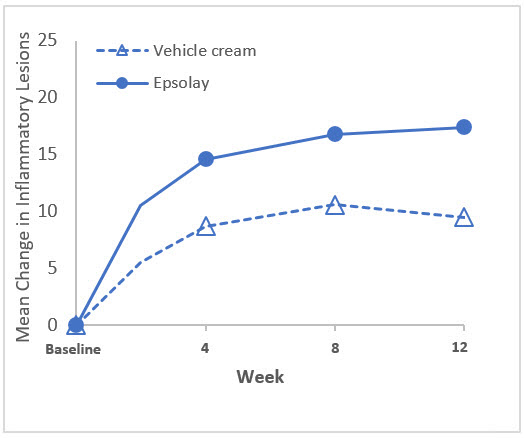
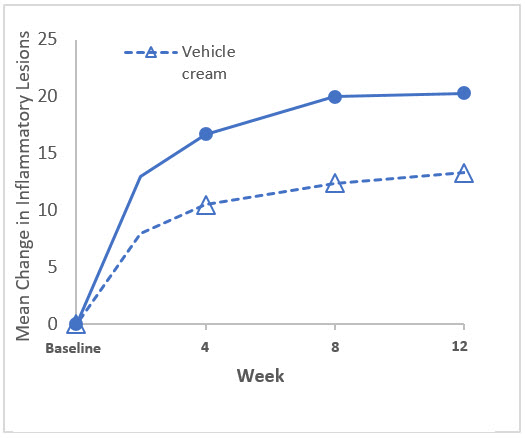
The co-primary efficacy endpoints in both trials were the proportion of subjects with treatment success at Week 12, defined as an IGA score of 0 ("clear") or 1 ("almost clear") with at least a two-grade reduction from baseline, and the absolute change from baseline in inflammatory lesion counts at Week 12. The results at Week 12 are presented in Table 3. EPSOLAY was more effective than vehicle cream on the co-primary efficacy endpoints starting from 4 weeks of treatment in both trials, see Figure 1 through Figure 4.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| EPSOLAY (N=243) | Vehicle (N=118) | EPSOLAY (N=250) | Vehicle (N=122) |
|
| IGA Treatment Success* | 47.4% | 20.7% | 49.2% | 28.2% |
| Difference from Vehicle | 26.7% | 21.0% | ||
| (95% CI) | (16.7%, 36.8%) | (10.7%, 31.3%) | ||
| Inflammatory Lesions | ||||
| Mean† Absolute Change | -17.4 | -9.5 | -20.3 | -13.3 |
| Difference from Vehicle | -7.9 | -6.9 | ||
| (95% CI) | (-10.0, -5.9) | (-9.0, -4.9) | ||
| Mean† Percent Change | -68.2% | -38.3% | -69.4% | -46.0% |
| Difference from Vehicle | -29.9% | -23.4% | ||
| (95% CI) | (-37.8%, -22.0%) | (-30.5%, -16.3%) | ||
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity
Inform patients that serious hypersensitivity reactions occurred with the use of benzoyl peroxide products. If a patient experiences a serious hypersensitivity reaction, instruct patient to discontinue EPSOLAY immediately and seek medical help [see Warnings and Precautions (5.1)].
Skin Irritation/Contact Dermatitis
Inform patients that EPSOLAY may cause irritation such as erythema, scaling, dryness, stinging or burning. Advise the patient to use a moisturizer for irritation [see Warnings and Precautions (5.2)].
Photosensitivity
Advise patients to minimize or avoid exposure to natural or artificial light (tanning beds or UVA/B treatment) and to use sun protective measures, if patients need to be outdoors while using EPSOLAY [see Warnings and Precautions (5.3)].
Administration Instructions
Advise patients to apply EPSOLAY exactly as directed in a thin layer, avoiding the eyes, lips and mouth and to wash hands immediately after application. Inform patients that EPSOLAY may bleach hair or colored fabric [see Dosage and Administration (2)].
Marketed by Sol-Gel Technologies Inc.
110 South Jefferson Rd.
Suite 203
Whippany, NJ 07981
Product of Canada
EPSOLAY is a trademark of Sol-Gel Technologies Ltd.
© 2021, Sol-Gel Technologies Ltd. All rights reserved.
| PATIENT INFORMATION EPSOLAY® (ep' soe lay) (benzoyl peroxide) cream |
|
|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Issued: 4/2022 |
| Important: EPSOLAY is for use on the skin only (topical). Do not use EPSOLAY in or on your mouth, eyes or vagina. | |
| What is EPSOLAY?
EPSOLAY is a prescription medicine used on the skin (topical) to treat adults with pimples and bumps caused by a condition called rosacea. It is not known if EPSOLAY is safe and effective in children. |
|
| Do not use EPSOLAY if you have had an allergic reaction to benzoyl peroxide or any of the ingredients in EPSOLAY. See the end of this leaflet for a complete list of ingredients in EPSOLAY. | |
Before using EPSOLAY, tell your healthcare provider about all of your medical conditions, including if you:
|
|
How should I use EPSOLAY?
|
|
What should I avoid while using EPSOLAY?
|
|
| What are the possible side effects of EPSOLAY? EPSOLAY may cause serious side effects, including:
These are not all the possible side effects with EPSOLAY. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store EPSOLAY?
|
|
| General information about the safe and effective use of EPSOLAY.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EPSOLAY for a condition for which it was not prescribed. Do not give EPSOLAY to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about EPSOLAY that is written for health professionals. |
|
| What are the ingredients in EPSOLAY?
Active ingredient: benzoyl peroxide Inactive ingredients: anhydrous citric acid, cetrimonium chloride, cetyl alcohol, cyclomethicone, edetate disodium, glycerin, hydrochloric acid, lactic acid, macrogol stearate Type I, mono and di-glycerides, phenoxyethanol, polyquaternium-7, purified water, silicon dioxide and sodium hydroxide. Marketed by Sol-Gel Technologies Inc., 110 South Jefferson Rd. Suite 203, Whippany, NJ 07981 Product of Canada EPSOLAY is a trademark of Sol-Gel Technologies, Ltd © 2021, Sol-Gel Technologies, Ltd. All rights reserved. For more information, go to www.EPSOLAY.com or call Sol-Gel Technologies Inc. at 1-866-SGT-AERS (748-2377). |
|
| EPSOLAY
benzoyl peroxide cream |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Sol-Gel Technologies Inc. (117528397) |
| Registrant - Sol-Gel Technologies Ltd. (532247350) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Groupe PARIMA Inc. | 252437850 | label(79167-005) , manufacture(79167-005) , pack(79167-005) | |