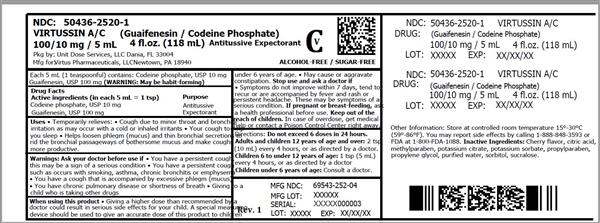
Uses
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with a cold or inhaled irritants
- your cough to help you sleep
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive.
Warnings
Ask your doctor before use if
- you have a persistent cough, this may be a sign of a serious condition
- you have a persistent cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- you have a cough that is accompanied by excessive phlegm (mucus)
- you have chronic pulmonary disease or shortness of breath
- giving to a child who is taking other drugs
When using this product
- giving a higher dose than recommended by a doctor could result in serious side effects for your child. A special measuring device should be used to give an accurate dose of this product to children under 6 years of age.
- may cause or aggravate constipation
Directions
- do not exceed 6 doses in 24 hours.
| Adults and children 12 years of age and over: | 2 tsp (10 mL) every 4 hours, or as directed by a doctor. |
| Children 6 to under 12 years of age: | 1 tsp (5 mL) every 4 hours, or as directed by a doctor. |
| Children under 6 years of age: | Consult a doctor. |
Other information
Store at controlled room temperature 15°-30°C (59°-86°F). You may report side effects by calling 1-888-848-3593 or FDA at 1-800-FDA-1088.