BIOCHEMISTRY PAIN RELIEF FOOT ACTIVE- benzyl alcohol, lidocaine hydrochloride liquid
Pure Source, LLC
----------
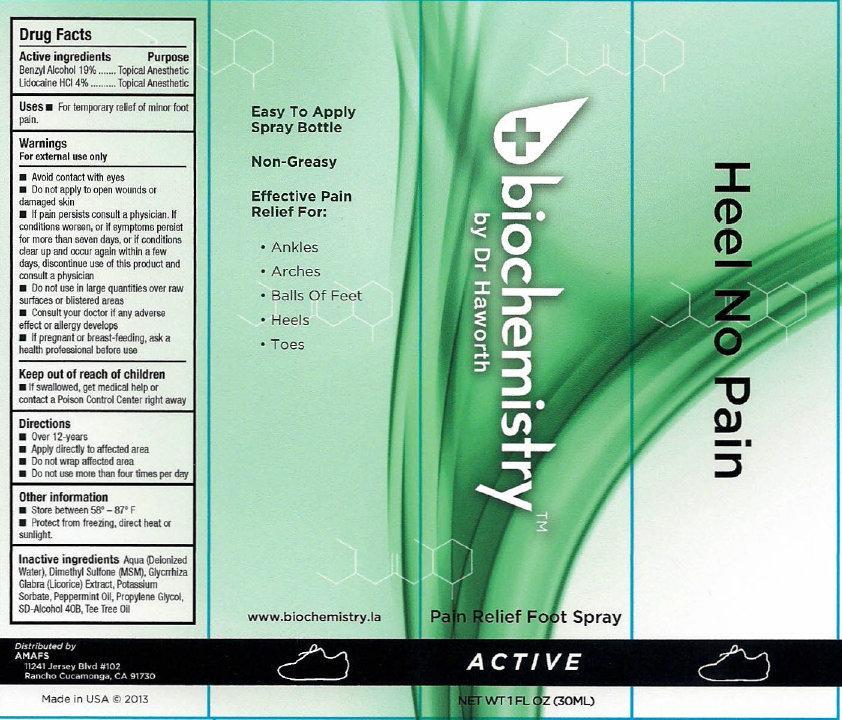
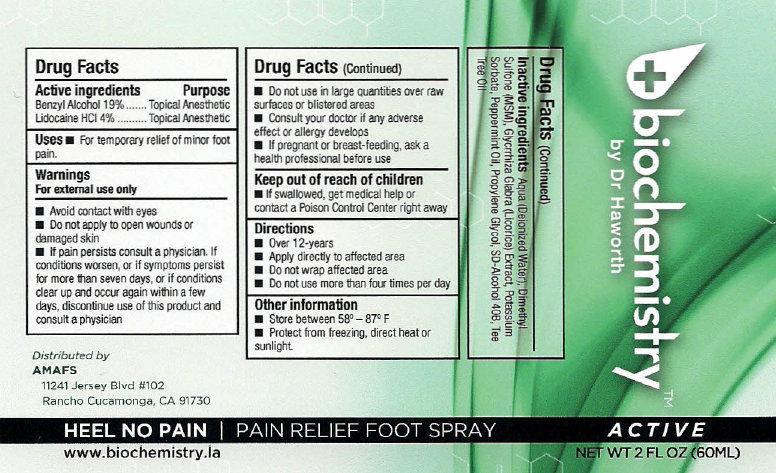
biochemistry PAIN RELIEF FOOT SPRAY ACTIVE
Active ingredients
Benzyl Alcohol 19% Lidocaline HCL 4%
Purpose
Topical Anesthetic
Uses
- For temporary relief of minor foot pain.
Warnings
For external use only
Avoid contact with eyes
- Do not apply to open wounds or damaged skin
- If pain persists consult a physician. If conditions worsens, or if symptoms persist for more than seven days, or if conditions clear up and occur again within a few days, discontinue use of this product and consult a physician
Do not use
in large quantities over raw surfaces or blistered areas
Consult your doctor
if any adverse effect or allergy develops
If pregnant or breast-feeding
ask a health professional before use
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away
Directions
- Over 12-years
- Apply directly to affected area
- Do not wrap affected area
- Do not use more than four times per day
Other information
- Store between 58 - 87 F
oo
- Protect from freezing, direct hear or sunlight.
Inactive ingredients
Aqua (Deionized Water), Dimethyl Sulfone (MSM), Glycyrrhiza Glabra (Licorice) Extract, Potassium Sorbate, Peppermint Oil, Propylene Glycol, SD-Alcohol 40B, Tea Tree Oil