INDICATIONS AND USAGE
-
Sumatriptan tablets, USP are indicated for the acute treatment of migraine with or without aura in adults.
Limitations of Use:- Use only if a clear diagnosis of migraine headache has been established. If a patient has no response to the first migraine attack treated with sumatriptan tablets, USP, reconsider the diagnosis of migraine before sumatriptan tablets, USP are administered to treat any subsequent attacks.
- Sumatriptan tablets, USP are not indicated for the prevention of migraine attacks.
- Safety and effectiveness of sumatriptan tablets, USP have not been established for cluster headache.
DOSAGE AND ADMINISTRATION
- 2.1 Dosing Information
The recommended dose of sumatriptan tablets is 25 mg, 50 mg, or 100 mg. Doses of 50 mg and 100 mg may provide a greater effect than the 25 mg dose, but doses of 100 mg may not provide a greater effect than the 50 mg dose. Higher doses may have a greater risk of adverse reactions [see Clinical Studies (14)].
2.2 Dosing in Patients With Hepatic Impairment
If the migraine has not resolved by 2 hours after taking sumatriptan tablets, or returns after a transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose is 200 mg in a 24-hour period.
Use after Sumatriptan Injection: If the migraine returns following an initial treatment with sumatriptan Injection, additional single sumatriptan tablets (up to 100 mg/day) may be given with an interval of at least 2 hours between tablet doses.
The safety of treating an average of more than 4 headaches in a 30-day period has not been established.If treatment is deemed advisable in the presence of mild to moderate hepatic impairment, the maximum single dose should not exceed 50 mg [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
DOSAGE FORMS AND STRENGTHS
25 mg Tablets: White to off-white, round, biconvex uncoated tablets, debossed with ‘C’ on one side and ‘32’ on other side.
50 mg Tablets: White to off-white, capsule shaped, biconvex uncoated tablets, debossed with ‘C’ on one side and ‘33’ on other side.
100 mg Tablets: White to off-white, capsule shaped, biconvex uncoated tablets, debossed with ‘C’ on one side and ‘34’ on other side.
CONTRAINDICATIONS
-
Sumatriptan tablets are contraindicated in patients with:
- Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal’s angina [see Warnings and Precautions (5.1)]
- Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Warnings and Precautions (5.2)]
- History of stroke or transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see Warnings and Precautions (5.4)]
- Peripheral vascular disease [see Warnings and Precautions (5.5)]
- Ischemic bowel disease [see Warnings and Precautions (5.5)]
- Uncontrolled hypertension [see Warnings and Precautions (5.8)]
- Recent use (i.e., within 24 hours) of ergotamine-containing medication, ergot-type medication (such as dihydroergotamine or methysergide), or another 5-hydroxytryptamine1 (5-HT1) agonist [see Drug Interactions (7.1,7.3)]
- Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent (within 2 weeks) use of an MAO-A inhibitor [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]
- Hypersensitivity to sumatriptan tablets (angioedema and anaphylaxis seen) [see Warnings and Precautions (5.9)]
- Severe hepatic impairment [see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)]
WARNINGS AND PRECAUTIONS
- 5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina
The use of sumatriptan tablets is contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of sumatriptan tablets. Some of these reactions occurred in patients without known CAD. Sumatriptan tablets may cause coronary artery vasospasm (Prinzmetal’s angina), even in patients without a history of CAD.
5.2 Arrhythmias
Perform a cardiovascular evaluation in triptan-naive patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving sumatriptan tablets. If there is evidence of CAD or coronary artery vasospasm, sumatriptan tablets are contraindicated. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first dose of sumatriptan tablets in a medically supervised setting and performing an electrocardiogram (ECG) immediately following administration of sumatriptan tablets. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of sumatriptan tablets.Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1 agonists. Discontinue sumatriptan tablets if these disturbances occur. Sumatriptan tablets are contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders.
5.3 Chest, Throat, Neck, and/or Jaw Pain/Tightness/PressureSensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with sumatriptan tablets and are usually non-cardiac in origin. However, perform a cardiac evaluation if these patients are at high cardiac risk. The use of sumatriptan tablets is contraindicated in patients with CAD and those with Prinzmetal’s variant angina.
5.4 Cerebrovascular EventsCerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, TIA). Discontinue sumatriptan tablets if a cerebrovascular event occurs.
5.5 Other Vasospasm Reactions
Before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, exclude other potentially serious neurological conditions. Sumatriptan tablets are contraindicated in patients with a history of stroke or TIA.Sumatriptan tablets may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1 agonist, rule out a vasospastic reaction before receiving additional sumatriptan tablets.
5.6 Medication Overuse Headache
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists have not been clearly established.Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
5.7 Serotonin SyndromeSerotonin syndrome may occur with sumatriptan tablets, particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Drug Interactions (7.4)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms usually occurs within minutes to hours of receiving a new or a greater dose of a serotonergic medication. Discontinue sumatriptan tablets if serotonin syndrome is suspected.
5.8 Increase in Blood PressureSignificant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT1 agonists, including patients without a history of hypertension. Monitor blood pressure in patients treated with sumatriptan. Sumatriptan tablets are contraindicated in patients with uncontrolled hypertension.
5.9 Anaphylactic/Anaphylactoid ReactionsAnaphylactic/anaphylactoid reactions have occurred in patients receiving sumatriptan. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. Sumatriptan tablets are contraindicated in patients with a history of hypersensitivity reaction to sumatriptan.
5.10 SeizuresSeizures have been reported following administration of sumatriptan. Some have occurred in patients with either a history of seizures or concurrent conditions predisposing to seizures. There are also reports in patients where no such predisposing factors are apparent. Sumatriptan tablets should be used with caution in patients with a history of epilepsy or conditions associated with a lowered seizure threshold.
ADVERSE REACTIONS
-
The following adverse reactions are discussed in more detail in other sections of the prescribing information:
- Myocardial ischemia, myocardial infarction, and Prinzmetal’s angina [see Warnings and Precautions (5.1)]
- Arrhythmias [see Warnings and Precautions (5.2)]
- Chest, throat, neck, and/or jaw pain/tightness/pressure [see Warnings and Precautions (5.3)]
- Cerebrovascular events [see Warnings and Precautions (5.4)]
- Other vasospasm reactions [see Warnings and Precautions (5.5)]
- Medication overuse headache [see Warnings and Precautions (5.6)]
- Serotonin syndrome [see Warnings and Precautions (5.7)]
- Increase in blood pressure [see Warnings and Precautions (5.8)]
- Hypersensitivity reactions [see Contraindications (4) and Warnings and Precautions (5.9)]
- Seizures [see Warnings and Precautions (5.10)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Table 1 lists adverse reactions that occurred in placebo-controlled clinical trials in patients who took at least 1 dose of study drug. Only treatment-emergent adverse reactions that occurred at a frequency of 2% or more in any group treated with sumatriptan tablets and that occurred at a frequency greater than the placebo group are included in Table 1.Table 1. Adverse Reactions Reported by at Least 2% of Patients Treated With Sumatriptan Tablets and at a Greater Frequency Than Placebo Adverse Reaction Percent of Patients Reporting Sumatriptan Tablets
25 mg
(n = 417)Sumatriptan Tablets
50 mg
(n = 771)Sumatriptan Tablets
100 mg
(n = 437)Placebo
(n = 309)Atypical sensations
Paresthesia (all types)
Sensation warm/cold5
3
36
5
26
3
34
2
2Pain and other pressure sensations 6 6 8 4 Chest - pain/tightness/ pressure and/or heaviness 1 2 2 1 Neck/throat/jaw -pain/ tightness/pressure <1 2 3 <1 Pain - location specified 2 1 1 1 Other - pressure/tightness/ heaviness 1 1 3 2 Neurological
Vertigo
<1
<1
2
<1Other
Malaise/fatigue
2
2
3
<1The incidence of adverse reactions in controlled clinical trials was not affected by gender or age of the patients. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
6.2 Postmarketing ExperienceThe following adverse reactions have been identified during postapproval use of sumatriptan tablets, sumatriptan nasal spray, and sumatriptan injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to sumatriptan or a combination of these factors.
Cardiovascular: Hypotension, palpitations.
Neurological: Dystonia, tremor.
DRUG INTERACTIONS
7.1 Ergot-Containing Drugs
Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) and sumatriptan tablets within 24 hours of each other is contraindicated.
7.2 Monoamine Oxidase-A Inhibitors
MAO-A inhibitors increase systemic exposure by 7-fold. Therefore, the use of sumatriptan tablets in patients receiving MAO-A inhibitors is contraindicated [see Clinical Pharmacology (12.3)].
7.3 Other 5-HT1 Agonists
Because their vasospastic effects may be additive, co-administration of sumatriptan tablets and other 5-HT1 agonists (e.g., triptans) within 24 hours of each other is contraindicated.
7.4 Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin Syndrome
Cases of serotonin syndrome have been reported during co-administration of triptans and SSRIs, SNRIs, TCAs, and MAO inhibitors [see Warnings and Precautions (5.7)].
USE IN SPECIFIC POPULATIONS
- 8.1 Pregnancy
Teratogenic Effects
8.3 Nursing Mothers
Pregnancy Category C:There are no adequate and well-controlled trials in pregnant women. In developmental toxicity studies in rats and rabbits, oral administration of sumatriptan to pregnant animals was associated with embryolethality, fetal abnormalities, and pup mortality. When administered by the intravenous route to pregnant rabbits, sumatriptan was embryolethal. Sumatriptan tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Oral administration of sumatriptan to pregnant rats during the period of organogenesis resulted in an increased incidence of fetal blood vessel (cervicothoracic and umbilical) abnormalities. The highest no-effect dose for embryofetal developmental toxicity in rats was 60 mg/kg/day, or approximately 3 times the maximum recommended human dose (MRHD) of 200 mg/day on a mg/m2 basis. Oral administration of sumatriptan to pregnant rabbits during the period of organogenesis resulted in increased incidences of embryolethality and fetal cervicothoracic vascular and skeletal abnormalities. Intravenous administration of sumatriptan to pregnant rabbits during the period of organogenesis resulted in an increased incidence of embryolethality. The highest oral and intravenous no-effect doses for developmental toxicity in rabbits were 15 (approximately 2 times the MRHD on a mg/m2 basis) and 0.75 mg/kg/day, respectively.
Oral administration of sumatriptan to rats prior to and throughout gestation resulted in embryofetal toxicity (decreased body weight, decreased ossification, increased incidence of skeletal abnormalities). The highest no-effect dose was 50 mg/kg/day, or approximately 2 times the MRHD on a mg/m2 basis. In offspring of pregnant rats treated orally with sumatriptan during organogenesis, there was a decrease in pup survival. The highest no-effect dose for this effect was 60 mg/kg/day, or approximately 3 times the MRHD on a mg/m2 basis. Oral treatment of pregnant rats with sumatriptan during the latter part of gestation and throughout lactation resulted in a decrease in pup survival. The highest no-effect dose for this finding was 100 mg/kg/day, or approximately 5 times the MRHD on a mg/m2 basis.
Sumatriptan is excreted in human milk following subcutaneous administration. Infant exposure to sumatriptan can be minimized by avoiding breastfeeding for 12 hours after treatment with sumatriptan tablets.
8.4 Pediatric UseSafety and effectiveness in pediatric patients have not been established. Sumatriptan tablets are not recommended for use in patients younger than 18 years of age.
8.5 Geriatric Use
Two controlled clinical trials evaluated sumatriptan nasal spray (5 to 20 mg) in 1,248 adolescent migraineurs aged 12 to 17 years who treated a single attack. The trials did not establish the efficacy of sumatriptan nasal spray compared with placebo in the treatment of migraine in adolescents. Adverse reactions observed in these clinical trials were similar in nature to those reported in clinical trials in adults.
Five controlled clinical trials (2 single-attack trials, 3 multiple-attack trials) evaluating oral sumatriptan (25 to 100 mg) in pediatric patients aged 12 to 17 years enrolled a total of 701 adolescent migraineurs. These trials did not establish the efficacy of oral sumatriptan compared with placebo in the treatment of migraine in adolescents. Adverse reactions observed in these clinical trials were similar in nature to those reported in clinical trials in adults. The frequency of all adverse reactions in these patients appeared to be both dose - and age-dependent, with younger patients reporting reactions more commonly than older adolescents.
Postmarketing experience documents that serious adverse reactions have occurred in the pediatric population after use of subcutaneous, oral, and/or intranasal sumatriptan. These reports include reactions similar in nature to those reported rarely in adults, including stroke, visual loss, and death. A myocardial infarction has been reported in a 14-year-old male following the use of oral sumatriptan; clinical signs occurred within 1 day of drug administration. Clinical data to determine the frequency of serious adverse reactions in pediatric patients who might receive subcutaneous, oral, or intranasal sumatriptan are not presently available.Clinical trials of sumatriptan tablets did not include sufficient numbers of patients aged 65 and older to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
A cardiovascular evaluation is recommended for geriatric patients who have other cardiovascular risk factors (e.g., diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving sumatriptan tablets [see Warnings and Precautions (5.1)].The maximum single dose in patients with mild to moderate hepatic impairment should not exceed 50 mg. Sumatriptan tablets are contraindicated in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
OVERDOSAGE
-
Patients in clinical trials (N = 670) received single oral doses of 140 to 300 mg without significant adverse reactions. Volunteers (N = 174) received single oral doses of 140 to 400 mg without serious adverse reactions.
Overdose in animals has been fatal and has been heralded by convulsions, tremor, paralysis, inactivity, ptosis, erythema of the extremities, abnormal respiration, cyanosis, ataxia, mydriasis, salivation, and lacrimation.
The elimination half-life of sumatriptan is approximately 2.5 hours [see Clinical Pharmacology (12.3)], and therefore monitoring of patients after overdose with sumatriptan tablets should continue for at least 12 hours or while symptoms or signs persist.
It is unknown what effect hemodialysis or peritoneal dialysis has on the serum concentrations of sumatriptan.
DESCRIPTION
-
Sumatriptan tablets, USP contain sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
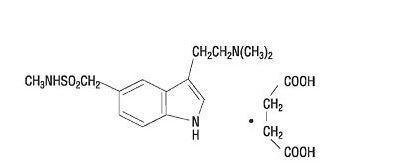
The molecular formula is C14H21N3O2S•C4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate USP is a white to off-white powder that is readily soluble in water and in saline.
Each sumatriptan tablet, USP for oral administration contains 35 mg, 70 mg, or 140 mg of sumatriptan succinate USP equivalent to 25 mg, 50 mg, or 100 mg of sumatriptan, respectively. Each tablet also contains the inactive ingredients croscarmellose sodium, dibasic calcium phosphate anhydrous, magnesium stearate, microcrystalline cellulose, polysorbate 80, and sodium bicarbonate.
CLINICAL PHARMACOLOGY
- 12.1 Mechanism of Action
Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine headache through agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels and sensory nerves of the trigeminal system, which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
12.2 PharmacodynamicsBlood Pressure: Significant elevation in blood pressure, including hypertensive crisis, has been reported in patients with and without a history of hypertension [see Warnings and Precautions (5.8)].
12.3 Pharmacokinetics
Peripheral (Small) Arteries: In healthy volunteers (N = 18), a trial evaluating the effects of sumatriptan on peripheral (small vessel) arterial reactivity failed to detect a clinically significant increase in peripheral resistance.
Heart Rate: Transient increases in blood pressure observed in some patients in clinical trials carried out during sumatriptan’s development as a treatment for migraine were not accompanied by any clinically significant changes in heart rate.Absorption and Bioavailability: The mean maximum concentration following oral dosing with 25 mg is 18 ng/mL (range: 7 to 47 ng/mL) and 51 ng/mL (range: 28 to 100 ng/mL) following oral dosing with 100 mg of sumatriptan. This compares with a Cmax of 5 and 16 ng/mL following dosing with a 5 and 20 mg intranasal dose, respectively. The mean Cmax following a 6 mg subcutaneous injection is 71 ng/mL (range: 49 to 110 ng/mL). The bioavailability is approximately 15%, primarily due to presystemic metabolism and partly due to incomplete absorption. The Cmax is similar during a migraine attack and during a migraine-free period, but the Tmax is slightly later during the attack, approximately 2.5 hours compared with 2 hours. When given as a single dose, sumatriptan displays dose proportionality in its extent of absorption (area under the curve [AUC]) over the dose range of 25 to 200 mg, but the Cmax after 100 mg is approximately 25% less than expected (based on the 25 mg dose).
A food effect trial involving administration of sumatriptan tablets 100 mg to healthy volunteers under fasting conditions and with a high-fat meal indicated that the Cmax and AUC were increased by 15% and 12%, respectively, when administered in the fed state.
Distribution: Protein binding, determined by equilibrium dialysis over the concentration range of 10 to 1,000 ng/mL is low, approximately 14% to 21%. The effect of sumatriptan on the protein binding of other drugs has not been evaluated. The apparent volume of distribution is 2.7 L/kg.
Metabolism:In vitro studies with human microsomes suggest that sumatriptan is metabolized by MAO, predominantly the A isoenzyme. Most of a radiolabeled dose of sumatriptan excreted in the urine is the major metabolite indole acetic acid (IAA) or the IAA glucuronide, both of which are inactive.
Elimination: The elimination half-life of sumatriptan is approximately 2.5 hours. Radiolabeled 14C-sumatriptan administered orally is largely renally excreted (about 60%) with about 40% found in the feces. Most of the radiolabeled compound excreted in the urine is the major metabolite, indole acetic acid (IAA), which is inactive, or the IAA glucuronide. Only 3% of the dose can be recovered as unchanged sumatriptan.
Special Populations:Age: The pharmacokinetics of sumatriptan in the elderly (mean age: 72 years, 2 males and 4 females) and in subjects with migraine (mean age: 38 years, 25 males and 155 females) were similar to that in healthy male subjects (mean age: 30 years).
Renal Impairment: The effect of renal impairment on the pharmacokinetics of sumatriptan has not been examined.
Hepatic Impairment: The liver plays an important role in the presystemic clearance of orally administered sumatriptan. Accordingly, the bioavailability of sumatriptan following oral administration may be markedly increased in patients with liver disease. In one small trial of patients with moderate liver impairment (n = 8) matched for sex, age, and weight with healthy subjects (n = 8), the hepatically-impaired patients had an approximately 70% increase in AUC and Cmax and a Tmax 40 minutes earlier compared to the healthy subjects.
The pharmacokinetics of sumatriptan in patients with severe hepatic impairment has not been studied. The use of sumatriptan tablets in this population is contraindicated [see Contraindications (4) and Use in Specific Populations (8.6)].
Gender: In a trial comparing females to males, no pharmacokinetic differences were observed between genders for AUC, Cmax, Tmax, and half-life.
Race: The systemic clearance and Cmax of subcutaneous sumatriptan were similar in black (n = 34) and Caucasian (n = 38) healthy male subjects. Oral sumatriptan has not been evaluated for race differences.
Drug Interaction Studies:Monoamine Oxidase-A Inhibitors: Treatment with MAO-A inhibitors generally leads to an increase of sumatriptan plasma levels [see Contraindications (4) and Drug Interactions (7.2)].
Due to gut and hepatic metabolic first-pass effects, the increase of systemic exposure after co-administration of an MAO-A inhibitor with oral sumatriptan is greater than after coadministration of the MAO inhibitors with subcutaneous sumatriptan.
In a trial of 14 healthy females, pretreatment with an MAO-A inhibitor decreased the clearance of subcutaneous sumatriptan, resulting in a 2-fold increase in the area under the sumatriptan plasma concentration-time curve (AUC), corresponding to a 40% increase in elimination half-life.
A small trial evaluating the effect of pretreatment with an MAO-A inhibitor on the bioavailability from a 25 mg oral sumatriptan tablet resulted in an approximately 7-fold increase in systemic exposure.
Alcohol: Alcohol consumed 30 minutes prior to sumatriptan ingestion had no effect on the pharmacokinetics of sumatriptan.
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: In carcinogenicity studies in mouse and rat, sumatriptan was administered orally for 78 and 104 weeks, respectively, at doses up to 160 mg/kg/day (the high dose in rat was reduced from 360 mg/kg/day during week 21). There was no evidence in either species of an increase in tumors related to sumatriptan administration. Plasma exposures (AUC) at the highest doses tested were 20 and 8 times that in humans at the maximum recommended human dose (MRHD) of 200 mg/day.
Mutagenesis: Sumatriptan was negative in in vitro (bacterial reverse mutation [Ames], gene cell mutation in Chinese hamster V79/HGPRT, chromosomal aberration in human lymphocytes) and in vivo (rat micronucleus) assays.
Impairment of Fertility: When sumatriptan (5, 50, 500 mg/kg/day) was administered orally to male and female rats prior to and throughout the mating period, there was a treatment-related decrease in fertility secondary to a decrease in mating in animals treated with doses greater than 5 mg/kg/day (less than the MRHD on a mg/m2 basis). It is not clear whether this finding was due to an effect on males or females or both.
13.2 Animal Toxicology and/or Pharmacology
Corneal Opacities: Dogs receiving oral sumatriptan developed corneal opacities and defects in the corneal epithelium. Corneal opacities were seen at the lowest dose tested, 2 mg/kg/day, and were present after 1 month of treatment. Defects in the corneal epithelium were noted in a 60-week study. Earlier examinations for these toxicities were not conducted and no-effect doses were not established. Plasma exposure at the lowest dose tested was approximately 2 times that in humans at the MRHD.
CLINICAL STUDIES
-
The efficacy of sumatriptan tablets in the acute treatment of migraine headaches was demonstrated in 3, randomized, double-blind, placebo-controlled trials. Patients enrolled in these 3 trials were predominately female (87%) and Caucasian (97%), with a mean age of 40 years (range of 18 to 65 years). Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed up to 4 hours after dosing. Associated symptoms such as nausea, photophobia, and phonophobia were also assessed. Maintenance of response was assessed for up to 24 hours postdose. A second dose of sumatriptan tablets or other medication was allowed 4 to 24 hours after the initial treatment for recurrent headache. Acetaminophen was offered to patients in Trials 2 and 3 beginning at 2 hours after initial treatment if the migraine pain had not improved or worsened. Additional medications were allowed 4 to 24 hours after the initial treatment for recurrent headache or as rescue in all 3 trials. The frequency and time to use of these additional treatments were also determined. In all trials, doses of 25, 50, and 100 mg were compared with placebo in the treatment of migraine attacks. In 1 trial, doses of 25, 50, and 100 mg were also compared with each other.
In all 3 trials, the percentage of patients achieving headache response 2 and 4 hours after treatment was significantly greater among patients receiving sumatriptan tablets at all doses compared with those who received placebo. In 1 of the 3 trials, there was a statistically significant greater percentage of patients with headache response at 2 and 4 hours in the 50 mg or 100 mg group when compared with the 25 mg dose groups. There were no statistically significant differences between the 50 mg and 100 mg dose groups in any trial. The results from the 3 controlled clinical trials are summarized in Table 2.Table 2. Percentage of Patients With Headache Response (Mild or No Headache) 2 and 4 Hours Following Treatment Sumatriptan Tablets
25 mg
2 hr 4 hrSumatriptan Tablets
50 mg
2 hr 4 hrSumatriptan Tablets
100 mg
2 hr 4 hrPlacebo
2 hr 4 hrTrial 1 52%a 67%a
(n = 298)61%a,b 78%a,b
(n = 296)62%a,b 79%a,b
(n = 296)27% 38%
(n = 94)Trial 2 52%a 70%a
(n = 66)50%a 68%a
(n = 62)56%a 71%a
(n = 66)26% 38%
(n = 65)Trial 3 52%a 65%a
(n = 48)54%a 72%a
(n = 46)57%a 78%a
(n = 46)17% 19%
(n = 47)a P<0.05 in comparison with placebo.
b P<0.05 in comparison with 25 mg.
The estimated probability of achieving an initial headache response over the 4 hours following treatment in pooled Trials 1, 2, and 3 is depicted in Figure 1.
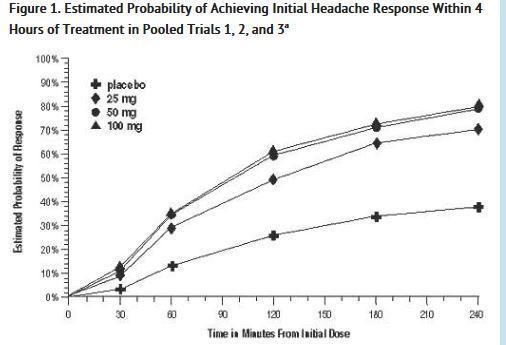
a The figure shows the probability over time of obtaining headache response (no or mild pain) following treatment with oral sumatriptan. The averages displayed are based on pooled data from the 3 clinical controlled trials providing evidence of efficacy. Kaplan-Meier plot with patients not achieving response and/or taking rescue within 240 minutes censored to 240 minutes.
For patients with migraine-associated nausea, photophobia, and/or phonophobia at baseline, there was a lower incidence of these symptoms at 2 hours (Trial 1) and at 4 hours (Trials 1, 2, and 3) following administration of sumatriptan tablets compared with placebo.
As early as 2 hours in Trials 2 and 3, or as early as 4 hours in Trial 1, through 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
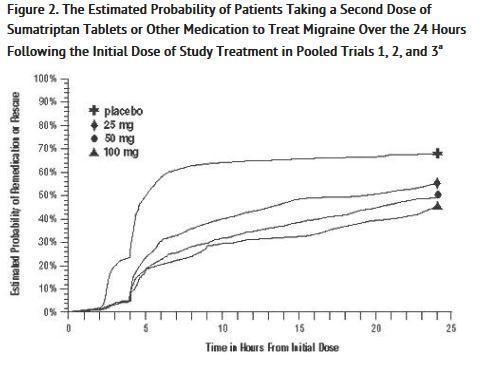
a Kaplan-Meier plot based on data obtained in the 3 clinical controlled trials providing evidence of efficacy with patients not using additional treatments censored to 24 hours. Plot also includes patients who had no response to the initial dose. No remedication was allowed within 2 hours postdose.
There is evidence that doses above 50 mg do not provide a greater effect than 50 mg. There was no evidence to suggest that treatment with sumatriptan tablets was associated with an increase in the severity of recurrent headaches. The efficacy of sumatriptan tablets was unaffected by presence of aura; duration of headache prior to treatment; gender, age, or weight of the subject; relationship to menses; or concomitant use of common migraine prophylactic drugs (e.g., beta-blockers, calcium channel blockers, tricyclic antidepressants). There were insufficient data to assess the impact of race on efficacy.
HOW SUPPLIED/STORAGE AND HANDLING
-
Sumatriptan Tablets USP, 25 mg, 50 mg, and 100 mg of sumatriptan (base) as the succinate.
Sumatriptan Tablets USP, 25 mg are white to off-white, round, biconvex uncoated tablets, debossed with ‘C’ on one side and ‘32’ on other side.
Unit-dose package of 9 Tablets NDC 65862-146-36
Sumatriptan Tablets USP, 50 mg are white to off-white, capsule shaped, biconvex uncoated tablets, debossed with ‘C’ on one side and ‘33’ on other side.
Unit-dose package of 9 Tablets NDC 65862-147-36
Sumatriptan Tablets USP, 100 mg are white to off-white, capsule shaped, biconvex uncoated tablets, debossed with ‘C’ on one side and ‘34’ on other side.
Unit-dose package of 9 Tablets NDC 65862-148-36
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Patient Information
-
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Risk of Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other Vasospasm-Related Events, Arrhythmias, and Cerebrovascular Events: Inform patients that sumatriptan tablets may cause serious cardiovascular side effects such as myocardial infarction or stroke. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, irregular heartbeat, significant rise in blood pressure, weakness, and slurring of speech, and should ask for medical advice if any indicative sign or symptoms are observed. Apprise patients of the importance of this follow-up [see Warnings and Precautions (5.1,5.2,5.4,5.5,5.8)].
Anaphylactic/Anaphylactoid Reactions: Inform patients that anaphylactic/anaphylactoid reactions have occurred in patients receiving sumatriptan tablets. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens [see Contraindications (4) and Warnings and Precautions (5.9)].
Concomitant Use With Other Triptans or Ergot Medications: Inform patients that use of sumatriptan tablets within 24 hours of another triptan or an ergot-type medication (including dihydroergotamine or methysergide) is contraindicated [see Contraindications (4),Drug Interactions (7.1,7.3)].
Serotonin Syndrome: Caution patients about the risk of serotonin syndrome with the use of sumatriptan tablets or other triptans, particularly during combined use with SSRIs, SNRIs, TCAs, and MAO inhibitors [see Warnings and Precautions (5.7),Drug Interactions (7.4)].
Medication Overuse Headache: Inform patients that use of acute migraine drugs for 10 or more days per month may lead to an exacerbation of headache and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary) [see Warnings and Precautions (5.6)].
Pregnancy: Inform patients that sumatriptan tablets should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus [see Use in Specific Populations (8.1)].
Nursing Mothers: Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [see Use in Specific Populations (8.3)].
Ability to Perform Complex Tasks: Treatment with sumatriptan tablets may cause somnolence and dizziness; instruct patients to evaluate their ability to perform complex tasks after administration of sumatriptan tablets.
-
Sumatriptan Tablets, USP
[Sumatriptan (soo ma TRIP tan)]
Read this Patient Information before you start taking sumatriptan tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about sumatriptan tablets?
Sumatriptan tablets can cause serious side effects, including:
Heart attack and other heart problems. Heart problems may lead to death.
Stop taking Sumatriptan tablets and get emergency medical help right away if you have any of the following symptoms of a heart attack:- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
Sumatriptan tablets are not for people with risk factors for heart disease unless a heart exam is done and shows no problem. You have a higher risk for heart disease if you:
- have high blood pressure
- have high cholesterol levels
- smoke
- are overweight
- have diabetes
- have a family history of heart disease
What are sumatriptan tablets?
Sumatriptan tablets are a prescription medicine used to treat acute migraine headaches with or without aura in adults.
Sumatriptan tablets are not used to treat other types of headaches such as hemiplegic (that make you unable to move on one side of your body) or basilar (rare form of migraine with aura) migraines.
Sumatriptan tablets are not used to prevent or decrease the number of migraine headaches you have.
It is not known if sumatriptan tablets are safe and effective to treat cluster headaches.
It is not known if sumatriptan tablets are safe and effective in children under 18 years of age.
Who should not take sumatriptan tablets?
Do not take sumatriptan tablets if you have:- heart problems or a history of heart problems
- narrowing of blood vessels to your legs, arms, stomach, or kidneys (peripheral vascular disease)
- uncontrolled high blood pressure
- severe liver problems
- hemiplegic migraines or basilar migraines. If you are not sure if you have these types of migraines, ask your healthcare provider.
- had a stroke, transient ischemic attacks (TIAs), or problems with your blood circulation
- taken any of the following medicines in the last 24 hours:
- almotriptan (AXERT®)
- eletriptan (RELPAX®)
- frovatriptan (FROVA®)
- naratriptan (AMERGE®)
- rizatriptan (MAXALT®, MAXALT-MLT®)
- sumatriptan and naproxen (TREXIMET®)
- ergotamines (CAFERGOT®, ERGOMAR®, MIGERGOT®)
- dihydroergotamine (D.H.E. 45®, MIGRANAL®)
Ask your healthcare provider if you are not sure if your medicine is listed above.
- an allergy to sumatriptan or any of the ingredients in sumatriptan tablets. See the end of this leaflet for a complete list of ingredients in sumatriptan tablets.
What should I tell my healthcare provider before taking sumatriptan tablets?
Before you take sumatriptan tablets, tell your healthcare provider about all of your medical conditions, including if you:- have high blood pressure
- have high cholesterol
- have diabetes
- smoke
- are overweight
- have heart problems or family history of heart problems or stroke
- have kidney problems
- have liver problems
- have had epilepsy or seizures
- are not using effective birth control
- become pregnant while taking sumatriptan tablets.
- are breastfeeding or plan to breastfeed. Sumatriptan passes into your breast milk and may harm your baby. Talk with your healthcare provider about the best way to feed your baby if you take sumatriptan tablets.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Sumatriptan tablets and certain other medicines can affect each other, causing serious side effects.
Especially tell your healthcare provider if you take anti-depressant medicines called:- selective serotonin reuptake inhibitors (SSRIs)
- serotonin norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants (TCAs)
- monoamine oxidase inhibitors (MAOIs)
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take sumatriptan tablets?- Certain people should take their first dose of sumatriptan tablets in their healthcare provider’s office or in another medical setting. Ask your healthcare provider if you should take your first dose in a medical setting.
- Take sumatriptan tablets exactly as your healthcare provider tells you to take them.
- Your healthcare provider may change your dose. Do not change your dose without first talking to your healthcare provider.
- Take sumatriptan tablets whole with water or other liquids.
- If you do not get any relief after your first tablet, do not take a second tablet without first talking with your healthcare provider.
- If your headache comes back or you only get some relief from your headache, you can take a second tablet 2 hours after the first tablet.
- Do not take more than 200 mg of sumatriptan tablets in a 24-hour period.
- If you take too much sumatriptan, call your healthcare provider or go to the nearest hospital emergency room right away.
- You should write down when you have headaches and when you take sumatriptan tablets so you can talk with your healthcare provider about how sumatriptan tablets are working for you.
What should I avoid while taking sumatriptan tablets?
Sumatriptan tablets can cause dizziness, weakness, or drowsiness. If you have these symptoms, do not drive a car, use machinery, or do anything where you need to be alert.
What are the possible side effects of sumatriptan tablets?
Sumatriptan tablets may cause serious side effects. See “What is the most important information I should know about sumatriptan tablets?”
These serious side effects include:- changes in color or sensation in your fingers and toes (Raynaud’s syndrome)
- stomach and intestinal problems (gastrointestinal and colonic ischemic events).
Symptoms of gastrointestinal and colonic ischemic events include:
- sudden or severe stomach pain
- stomach pain after meals
- weight loss
- nausea or vomiting
- constipation or diarrhea
- bloody diarrhea
- fever
- problems with blood circulation to your legs and feet (peripheral vascular ischemia). Symptoms of peripheral vascular ischemia include:
- cramping and pain in your legs or hips
- feeling of heaviness or tightness in your leg muscles
- burning or aching pain in your feet or toes while resting
- numbness, tingling, or weakness in your legs
- cold feeling or color changes in 1 or both legs or feet
- hives (itchy bumps); swelling of your tongue, mouth, or throat
- medication overuse headaches. Some people who use too many sumatriptan tablets may have worse headaches (medication overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with sumatriptan tablets.
- serotonin syndrome. Serotonin syndrome is a rare but serious problem that can happen in people using sumatriptan tablets, especially if sumatriptan tablets are used with anti-depressant medicines called SSRIs or SNRIs.
Call your healthcare provider right away if you have any of the following symptoms of serotonin syndrome:
- mental changes such as seeing things that are not there (hallucinations), agitation, or coma
- fast heartbeat
- changes in blood pressure
- high body temperature
- tight muscles
- trouble walking
- seizures. Seizures have happened in people taking sumatriptan tablets who have never had seizures before. Talk with your healthcare provider about your chance of having seizures while you take sumatriptan tablets.
The most common side effects of sumatriptan tablets include:
- tingling or numbness in your fingers or toes
- warm or cold feeling
- feeling weak, drowsy, or tired
- pain, discomfort, or stiffness in your neck, throat, jaw, or chest
- dizziness
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of sumatriptan tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store sumatriptan tablets?
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Keep sumatriptan tablets and all medicines out of the reach of children.
General information about the safe and effective use of sumatriptan tablets.
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use sumatriptan tablets for a condition for which it was not prescribed. Do not give sumatriptan tablets to other people, even if they have the same symptoms you have. They may harm them.
This Patient Information leaflet summarizes the most important information about sumatriptan tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about sumatriptan tablets that is written for healthcare professionals.
For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876.
What are the ingredients in sumatriptan tablets?
Active ingredient: sumatriptan succinate
Inactive ingredients: croscarmellose sodium, dibasic calcium phosphate anhydrous, magnesium stearate, microcrystalline cellulose, polysorbate 80, and sodium bicarbonate.
This Patient Information has been approved by the U.S. Food and Drug Administration.
All brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
Manufactured for:
Aurobindo Pharma USA, Inc.
2400 Route 130 North
Dayton, NJ 08810
Manufactured by:
Aurobindo Pharma Limited
Hyderabad–500 072, India
-
