PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 65841-759-01 in bottle of 100 Capsules
Omeprazole Delayed-release Capsules USP, 10 mg
Rx only
100 Capsules

NDC 65841-760-01 in bottle of 100 Capsules
Omeprazole Delayed-release Capsules USP, 20 mg
Rx only
100 Capsules

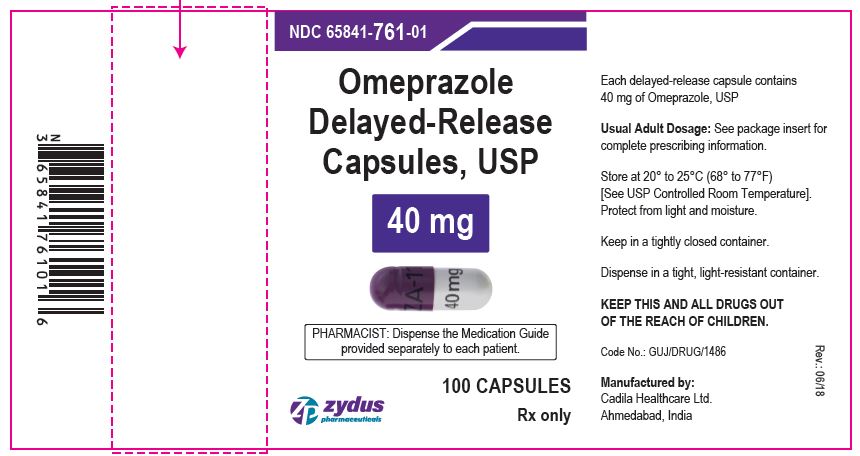
NDC 65841-761-01 in bottle of 100 Capsules
Omeprazole Delayed-release Capsules USP, 40 mg
Rx only
100 Capsules