Uses
- cures most athlete's foot (tinea pedis)
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- relieves itching, burning, cracking and scaling which accompany these conditions
Warnings
For external use only
Directions
- adults and children 12 years and older
- use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying
-
for athlete's foot wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
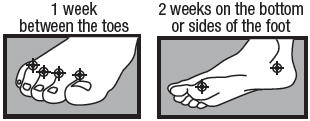
- between the toes only: apply twice a day (morning and night) for 1 week or as directed by a doctor.
- on the bottom or sides of the foot: apply twice a day (morning and night) for 2 weeks or as directed by a doctor.
- for jock itch and ringworm: apply once a day (morning or night) for 1 week or as directed by a doctor.
- wash hands after each use
- children under 12 years: ask a doctor
Other information
- do not use if seal on tube is broken or is not visible
- store at controlled room temperature 20°-25°C (68°-77°F)
- see carton or tube crimp for lot number and expiration date
