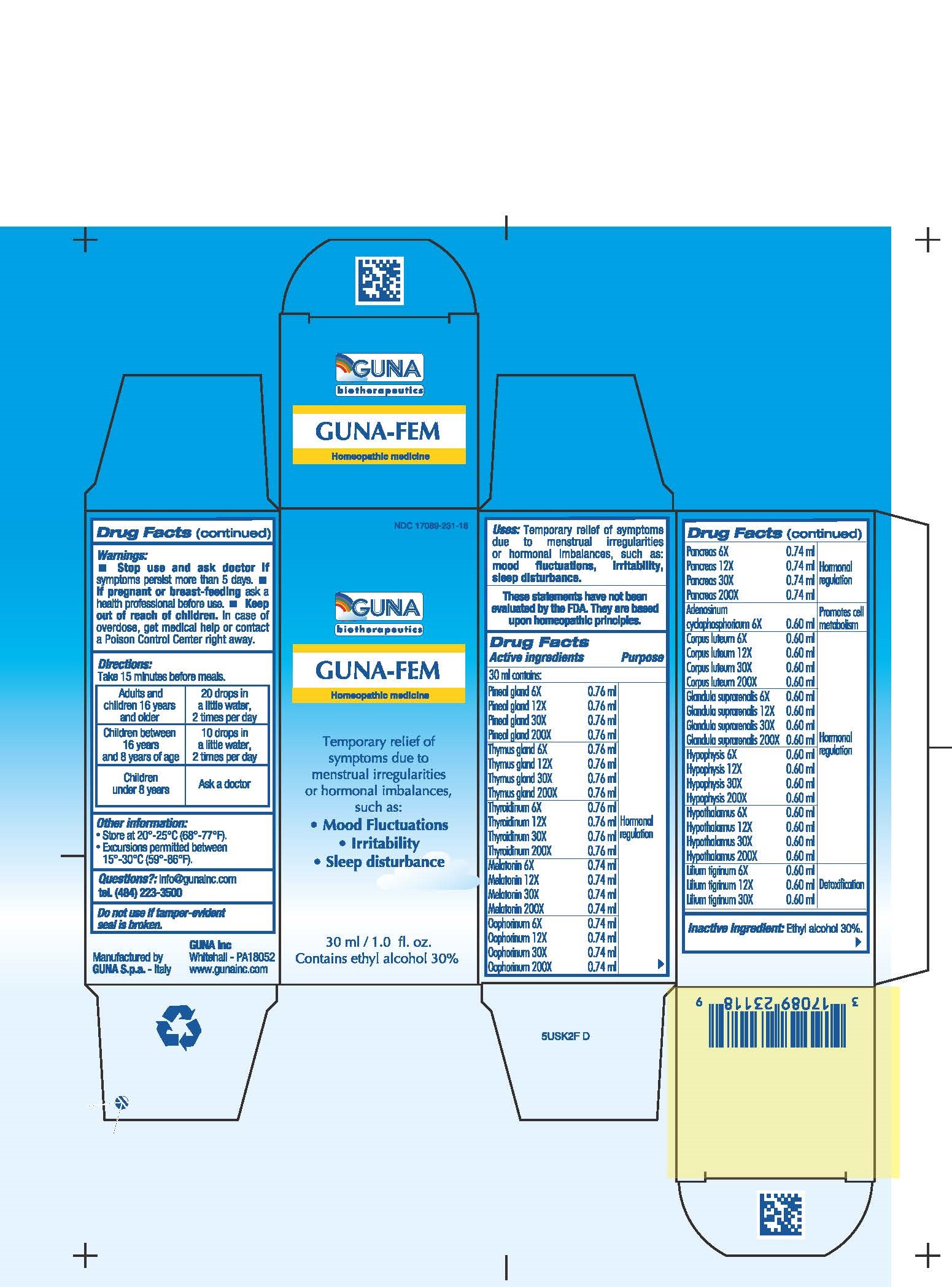
ACTIVE INGREDIENTS/PURPOSE
ADENOSINUM CICLOPHOSPHORICUM 6X PROMOTES CELL METABOLISM
CORPUS LUTEUM 6X 12X 30X, 200X HORMONAL REGULATION
GLANDULA SUPRARENALIS 6X, 12X, 30X, 200X HORMONAL REGULATION
HYPOPHYSIS 6X, 12X, 30X, 200X HORMONAL REGULATION
HYPOTHALAMUS 6X, 12X, 30X, 200X HORMONAL REGULATION
LILIUM TIGRINUM 6X, 12X, 30X DETOXIFICATION
MELATONIN 6X, 12X, 30X, 200X HORMONAL REGULATION
OOPHORINUM 6X, 12X, 30X, 200X HORMONAL REGULATION
PANCREAS 6X, 12X, 30X, 200X HORMONAL REGULATION
PINEAL GLAND 6X, 12X, 30X, 200X HORMONAL REGULATION
THYMUS GLAND 6X, 12X, 30X, 200X HORMONAL REGULATION
THYROIDINUM 6X, 12X, 30X, 200X HORMONAL REGULATION
USES
Temporary relief of symptoms due to menstrual irregularities or hormonal imbalances, such as:
- Mood Fluctuations
- Irritability
- Sleep disturbance
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Contains ethyl alcohol 30%