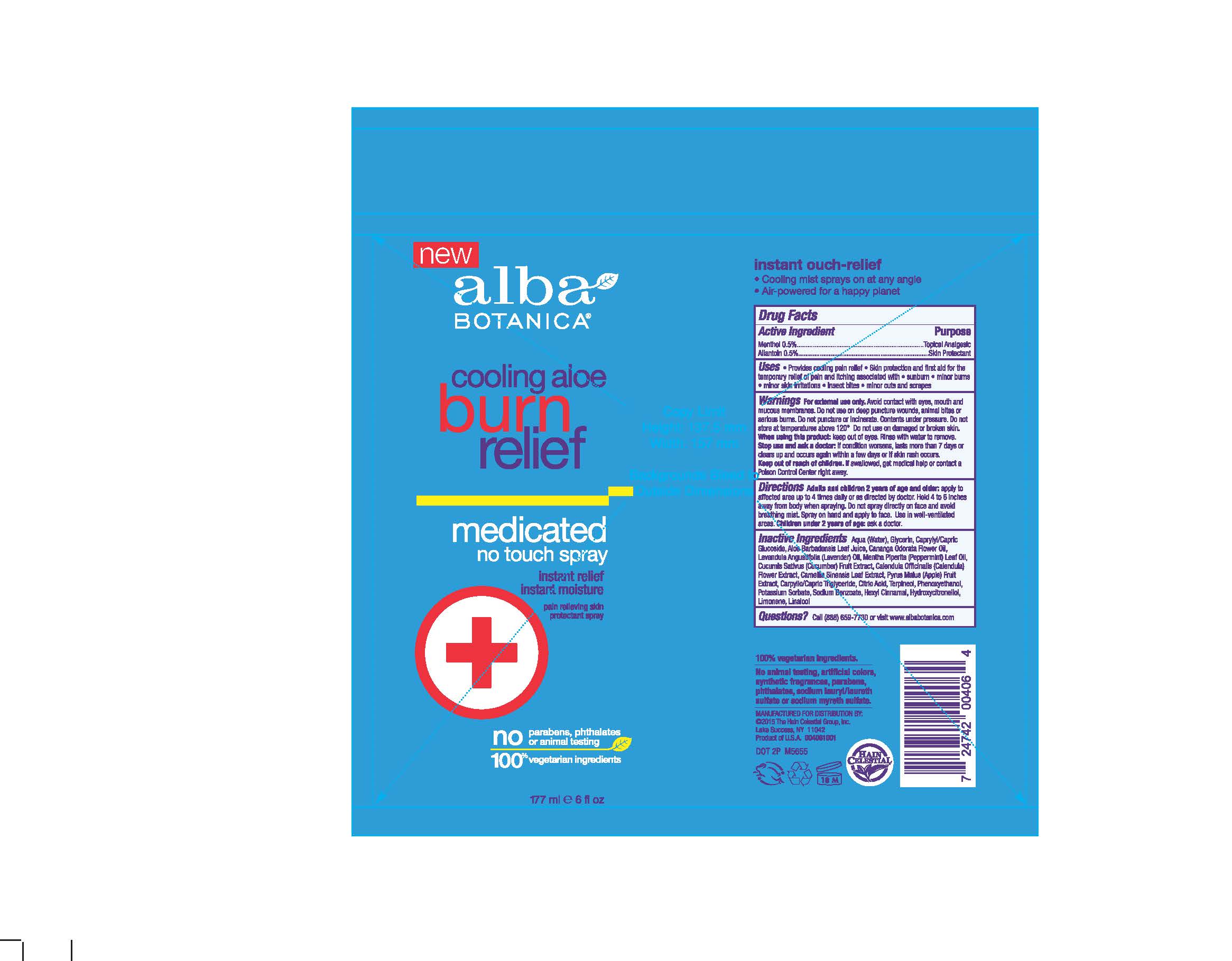
- Provides cooling pain relieve
- Skin protection and first aid for the temporary relief of pain and itching associated with sunburn, minor burns, minor skin irritations, insect bites, minor cuts and scrapes
For external use only. Avoid contact with eyes, mouth and mucous membranes. Do not use on deep puncture wounds, animal bites or serious burns. Do not puncture or incinerate. Contents under preassure .Do not store at temperature above 120°. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask doctor if condition worsens, lasts more than 7days or clears up and occurs again in within a few days or if skin rash occurs.
Adults and children 2 years old and older: apply to affected area up to 4 times daily or as directed by doctor. Hold 4-6 inches away from body when spraying. Do not spray directly on face and avoid breathing mist. Sprayon hand and apply to face.Use in well-ventiled area.
Children under 2 years of age : ask a doctor.
Aquq (Water),Glycerin, Caprylyl/Capric Glucoside, Aloe Barbadensis Leaf Juice, Cananga Odarata Flower Oil, Mentha Piperita (Peppermint) Oil, Cucumis Sativus (Cucumber) Fruit Extract, Calendula Officinalis Flower Extract, Camellia Sinensis Leaf Extract, Pyrus Malus (Apple) Fruit Extract, Caprylic/Capric Trigluceride, Citric Acid, Terpineol, Phenoxyethanol, Potassium Sorbate, Sodium Benzoate, Hexyl Cinnamal, Hydroxycitronellol, Limonene, Linalool.