ROOSIN BURN- lidocaine hydrochloride cream
ROOSIN MEDICAL CO., LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
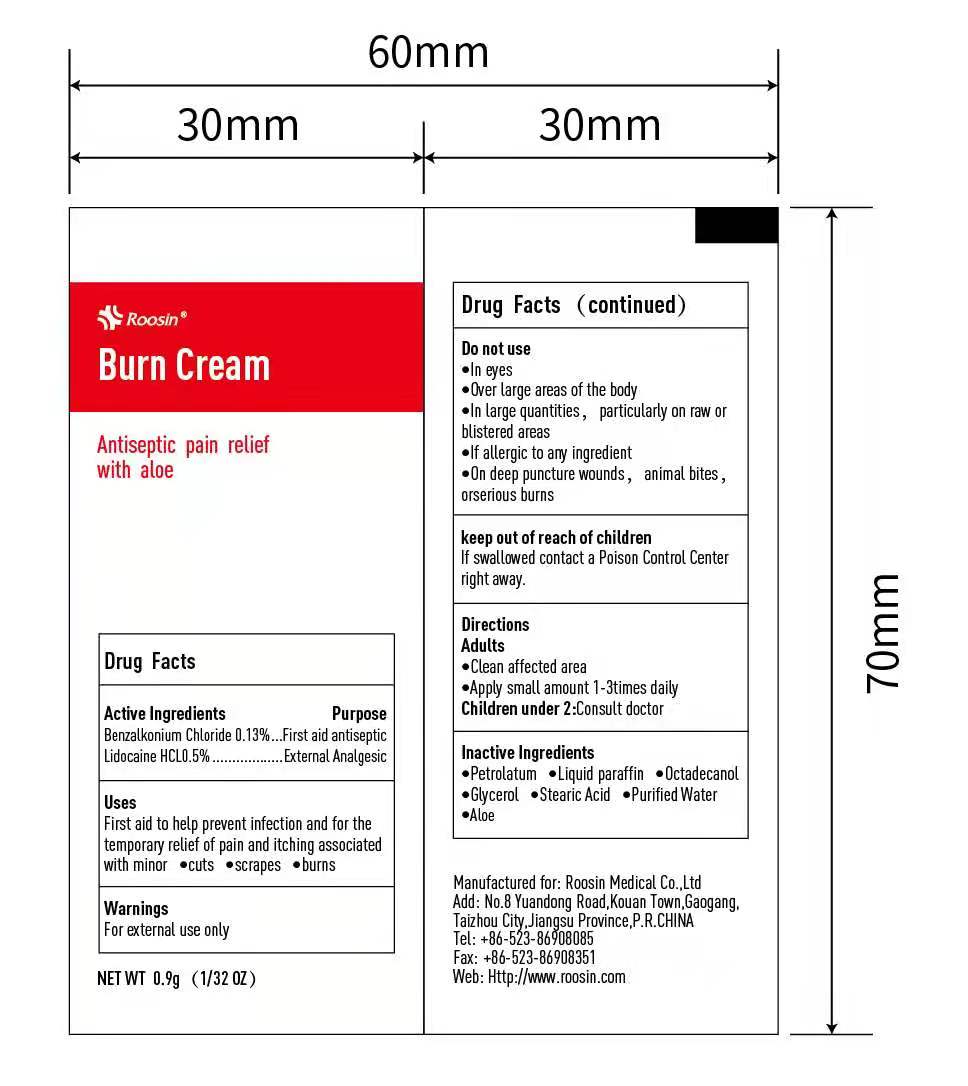
Active Ingredient
Banzalkonium Chloride 0.13%
Lidocaine Chloride 0.5%
Purpose
First Aid Antiseptic
External Analgesic
Antiseptic and antipyrotic
Use
First aid to help prevent infection and for temporary relief of pain and itching associated with minor cuts scrapes burns
WARNINGS
For external use only
Do not use
- In eyes
- Over large areas of the body
- For more than one week unless directed by a doctor
- If you are allergic to any of the ingredients
- On deep puncture wounds, animal bites, or serious burns
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away
Directions
Clean affected area
Apply a small amount 1-3 times daily
Children under 2: Consult doctor
Inactive ingredients
Petrolactum, Liquid Paraffin, Octadecanol, Glycerol, Stearic Acid, Purified Water, Aloe