Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Directions
- chew or crush tablets completely before swallowing.
| adults and children 6 years and over | chew 2 tablets daily; not more than 2 tablets in 24 hours |
| children 2 to under 6 years of age | chew 1 tablet daily; not more than 1 tablet in 24 hours |
| children under 2 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
- phenylketonurics: contains phenylalanine 1.25 mg per tablet.
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 68° to 77°F (20° to 25°C).
Inactive ingredients
aspartame, citric acid anhydrous, colloidal silicon dioxide, flavor, magnesium stearate, mannitol, microcrystalline cellulose, sodium starch glycolate, stearic acid
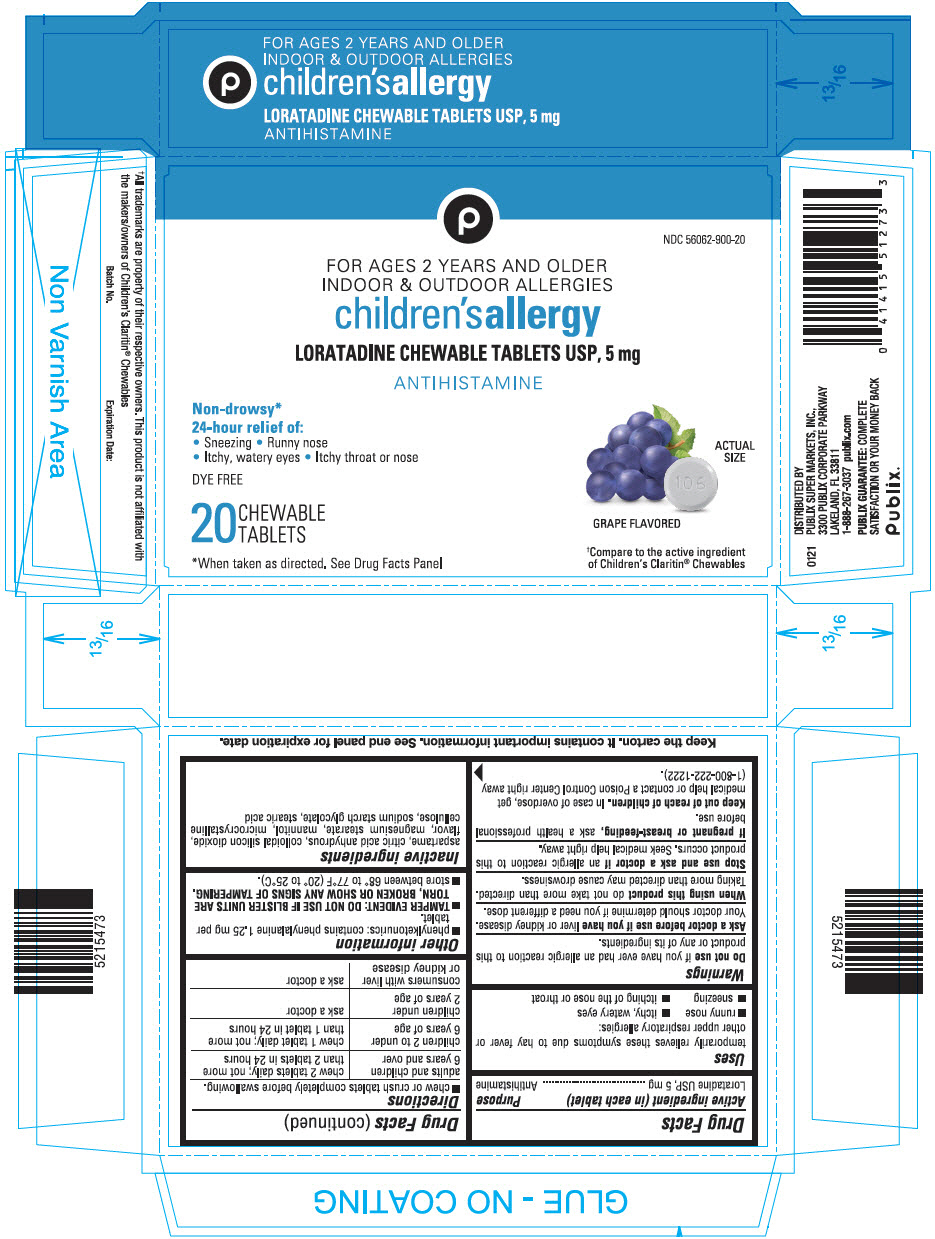
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Blister Pack Carton
NDC 56062-900-20
FOR AGES 2 YEARS AND OLDER
INDOOR & OUTDOOR ALLERGIES
children's allergy
LORATADINE CHEWABLE TABLETS USP, 5 mg
ANTIHISTAMINE
Non-drowsy*
24-hour relief of:
• Sneezing • Runny nose
• Itchy, watery eyes • Itchy throat or nose
DYE FREE
ACTUAL
SIZE
20
CHEWABLE
TABLETS
GRAPE FLAVORED
*When taken as directed. See Drug Facts Panel
†Compare to the active ingredient
of Children's Claritin® Chewables