FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
VANDAZOLE® is indicated in the treatment of bacterial vaginosis (formerly referred to as Haemophilus vaginitis, Gardnerella vaginitis, nonspecific vaginitis, Corynebacterium vaginitis, or anaerobic vaginosis) in post-menarchal females.
2 DOSAGE AND ADMINISTRATION
The recommended dose is one applicator full of VANDAZOLE, (approximately 5 grams of gel containing approximately 37.5 mg of metronidazole) administered intravaginally once a day for 5 days. For once a day dosing, VANDAZOLE should be administered at bedtime [see Patient Counseling Information (17.4)].
Not for ophthalmic, dermal, or oral use.
3 DOSAGE FORMS AND STRENGTHS
Gel, 0.75%. VANDAZOLE is a clear, colorless to yellow gel in a 70 g tube, supplied with 5 vaginal applicators. Each applicator delivers approximately 5 g of gel containing 37.5 mg of metronidazole, USP.
4 CONTRAINDICATIONS
4.1 Hypersensitivity
The use of VANDAZOLE is contraindicated in patients with a history of hypersensitivity to metronidazole, other nitroimidazole derivatives, or parabens. Reported reactions include urticaria; erythematous rash; Stevens-Johnson Syndrome, toxic epidermal necrolysis, flushing; nasal congestion; dryness of the mouth, vagina, or vulva; fever; pruritus; fleeting joint pains [see Adverse Reactions (6.2)].
4.2 Psychotic Reaction with Disulfiram
Use of oral metronidazole is associated with psychotic reactions in alcoholic patients who were using disulfiram concurrently. Do not administer VANDAZOLE to patients who have taken disulfiram within the last two weeks [see Adverse Reactions (6.2)].
4.3 Interaction with Alcohol
Use of oral metronidazole is associated with a disulfiram-like reaction to alcohol, including abdominal cramps, nausea, vomiting, headaches, and flushing [see Adverse Reactions (6.2)]. Discontinue alcohol consumption during and for at least three days after therapy with VANDAZOLE.
5 WARNINGS AND PRECAUTIONS
5.1 Central and Peripheral Nervous System Effects
Use of oral or intravenous metronidazole is associated with convulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity [see Adverse Reactions (6.2)]. VANDAZOLE should be administered with caution to patients with central nervous system diseases. Discontinue VANDAZOLE promptly if a patient develops abnormal neurologic signs.
5.2 Carcinogenicity in Animals
Metronidazole has been shown to be carcinogenic in mice and rats [see Nonclinical Toxicology (13.1)]. Unnecessary use of metronidazole should be avoided. Use of VANDAZOLE should be reserved for the treatment of bacterial vaginosis [see Indications and Usage (1)].
5.3 Interference with Laboratory Tests
Metronidazole may interfere with certain types of determinations of serum chemistry values, such as aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactate dehydrogenase (LDH), triglycerides, and glucose hexokinase. Values of zero may be observed. All of the assays in which interference has been reported involve enzymatic coupling of the assay to oxidation-reduction of nicotinamide-adenine dinucleotides (NAD + NADH). Interference is due to the similarity in absorbance peaks of NADH (340 nm) and metronidazole (322 nm) at pH 7. Consider postponing chemistry laboratory tests until treatment with VANDAZOLE is completed.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to VANDAZOLE compared to another formulation of vaginal metronidazole in 220 women in a single trial. The population was non-pregnant females (age range 18 to 72 years, the mean was 33 years +/- 11 years) with bacterial vaginosis. The racial demographic of those enrolled was 71 (32%) of White, 143 (65%) of Black, 3 (1%) of Hispanic, 2 (1%) of Asian, and 1 (0%) of other. Patients administered an applicator full of VANDAZOLE intravaginally once daily at bedtime for 5 days.
There were no deaths or serious adverse reactions related to drug therapy in the clinical trial. VANDAZOLE was discontinued in 5 patients (2.3%) due to adverse reactions.
The incidence of all adverse reactions in VANDAZOLE-treated patients was 42% (92/220). Adverse reactions occurring in ≥ 1% of patients were: fungal infection* (12%), headache (7%), pruritus (6%), abdominal pain (5%), nausea (3%), dysmenorrhea (3%), pharyngitis (2%), rash (1%), infection (1%), diarrhea (1%), breast pain (1%), and metrorrhagia (1%).
* Known or previously unrecognized vaginal candidiasis may present more prominent symptoms during therapy with VANDAZOLE. Approximately 10% of patients treated with VANDAZOLE developed Candida vaginitis during or immediately after therapy.
Additional uncommon events, reported by < 1% of those women treated with VANDAZOLE included:
|
General: |
allergic reaction, back pain, flu syndrome, mucous membrane disorder, pain |
|
Gastrointestinal: |
anorexia, constipation, dyspepsia, flatulence, gingivitis, vomiting |
|
Nervous System: |
depression, dizziness, insomnia |
|
Respiratory System: |
asthma, rhinitis |
|
Skin and Appendages: |
acne, sweating, urticaria |
|
Urogenital System: |
breast enlargement, dysuria, female lactation, labial edema, leucorrhea, menorrhagia, pyelonephritis, salpingitis, urinary frequency, urinary tract infection, vaginitis, vulvovaginal disorder |
6.2 Other Metronidazole Formulations
Other Vaginal Formulations
Other reactions that have been reported in association with the use of other formulations of metronidazole vaginal gel include: unusual taste and decreased appetite.
Topical (Dermal) Formulations
Other reactions that have been reported in association with the use of topical (dermal) formulations of metronidazole include skin irritation, transient skin erythema, and mild skin dryness and burning. None of these adverse reactions exceeded an incidence of 2% of patients.
Oral and Parenteral Formulations
The following adverse reactions and altered laboratory tests have been reported with the oral or parenteral use of metronidazole:
Cardiovascular: Flattening of the T-wave may be seen in electrocardiographic tracings.
Nervous System: The most serious adverse reactions reported in patients treated with metronidazole have been convulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity. In addition, patients have reported syncope, vertigo, incoordination, ataxia, confusion, dysarthria, irritability, depression, weakness, and insomnia [see Warnings and Precautions (5.1)].
Gastrointestinal: Abdominal discomfort, nausea, vomiting, diarrhea, an unpleasant metallic taste, anorexia, epigastric distress, abdominal cramping, constipation, “furry” tongue, glossitis, stomatitis, pancreatitis, and modification of taste of alcoholic beverages.
Genitourinary: Overgrowth of Candida in the vagina, dyspareunia, decreased libido, proctitis.
Hematopoietic: Reversible neutropenia, reversible thrombocytopenia.
Hypersensitivity Reactions: Urticaria; erythematous rash; Stevens-Johnson Syndrome, toxic epidermal necrolysis, flushing; nasal congestion; dryness of the mouth, vagina, or vulva; fever; pruritus; fleeting joint pains [see Contraindications (4.1)].
Renal: Dysuria, cystitis, polyuria, incontinence, a sense of pelvic pressure, darkened urine.
7 DRUG INTERACTIONS
The intravaginal administration of a single 5 gram dose of VANDAZOLE results in relatively lower mean systemic exposure to metronidazole that is approximately 2% to 5% of that achieved following a 500 mg oral dose of metronidazole [see Clinical Pharmacology (12.3)]. The following drug interactions were reported for oral metronidazole.
7.1 Disulfiram
Use of oral metronidazole has been associated with psychotic reactions in alcoholic patients who are using disulfiram concurrently. VANDAZOLE should not be used by patients who have taken disulfiram within the last two weeks [see Contraindications (4.2)].
7.2 Alcoholic Beverages
Use of oral metronidazole has been associated with a disulfiram-like reaction (abdominal cramps, nausea, vomiting, headaches, and flushing) to alcohol. Alcoholic beverages and preparations containing ethanol or propylene glycol should not be consumed during and for at least three days after VANDAZOLE therapy [see Contraindications (4.3)].
7.3 Coumarin and Other Oral Anticoagulants
Use of oral metronidazole has been reported to potentiate the anticoagulant effect of warfarin and other coumarin anticoagulants, resulting in a prolongation of prothrombin time. This possible drug interaction should be considered when VANDAZOLE is prescribed for patients on this type of anticoagulant therapy. In patients on oral anticoagulants, consider monitoring prothrombin time, international normalized ratio (INR), and other coagulation parameters while on VANDAZOLE.
7.4 Lithium
Short-term use of oral metronidazole has been associated with elevation of serum lithium concentrations and, in a few cases signs of lithium toxicity, in patients stabilized on relatively high doses of lithium. Use VANDAZOLE with caution in patients treated with lithium and consider monitoring lithium serum concentrations while on VANDAZOLE.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data on metronidazole use in pregnant women from published cohort studies, case-control studies, case series, meta analyses, and case reports over several decades have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, no adverse developmental effects were demonstrated when oral metronidazole was administered to mice at doses up to six times the recommended human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Published case-control studies, cohort studies, meta analyses, case series, and case reports over several decades have not established a risk with metronidazole use in pregnancy and major birth defects, miscarriage, or adverse maternal or fetal outcomes. Many studies included first trimester exposures. One study showed an increased risk of cleft lip, with or without cleft palate, in infants exposed to metronidazole in-utero; however, these findings were not confirmed. Most studies did not show an increased risk for congenital anomalies or other adverse fetal outcomes following metronidazole exposure during pregnancy. Studies conducted to assess the risk of infant cancer following systemic metronidazole exposure during pregnancy did not show an increased risk; however, the ability of these studies to detect such a signal was limited.
Animal Data
Animal studies have shown that metronidazole crosses the placental barrier and enters the fetal circulation rapidly. Oral metronidazole reproductive toxicity studies have been performed in mice at doses up to six times the recommended human dose based on body surface area comparisons and have revealed no evidence of harm to the fetus. However, in a single small study where the drug was administered intraperitoneally, some intrauterine deaths were observed.
8.2 Lactation
Risk Summary
There are no data on the presence of metronidazole in human milk following intravaginal administration. Metronidazole is present in human milk following oral metronidazole administration at concentrations similar to those found in plasma (see Data). The metronidazole vaginal gel achieves 2% of the mean maximum serum concentration of a 500 mg oral metronidazole dose [see Clinical Pharmacology (12.3)]. The published literature reports no adverse effects in infants exposed through breastmilk to maternal orally administered metronidazole. There are no data on the effects on milk production.
Animal studies have shown the potential for tumorigenicity after oral metronidazole was administered chronically to rats and mice [see Nonclinical Toxicology (13.1)]. The clinical relevance of these findings is unclear. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VANDAZOLE, and any potential adverse effects on the breastfed child from VANDAZOLE or from the underlying maternal condition. Alternatively, a lactating patient may interrupt breastfeeding and choose to pump and discard breastmilk during treatment with VANDAZOLE and for 48 hours after the last dose and feed her infant previously stored human milk or formula.
Data
In a study of lactating women receiving oral metronidazole 600 mg (n=11) or 1200 mg (n=4) daily, mean maternal plasma concentrations were 5.0 and 12.5 mcg/mL, respectively, within 2 hours following administration; the milk: maternal plasma ratio was approximately 1.
8.4 Pediatric Use
The safety and efficacy of VANDAZOLE in the treatment of bacterial vaginosis in post-menarchal females have been established on the extrapolation of clinical trial data from adult females.
The safety and efficacy of VANDAZOLE in pre-menarchal females have not been established.
8.5 Geriatric Use
Clinical studies with VANDAZOLE did not include sufficient numbers of subjects 65 years of age or older to determine whether they respond differently than younger subjects. Other reported clinical experience in using metronidazole gel, 1% has not identified differences in responses between elderly and younger patients.
10 OVERDOSAGE
There is no human experience with overdosage of metronidazole vaginal gel. Vaginally applied metronidazole gel, 0.75% could be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5) and Adverse Reactions (6.2)].
11 DESCRIPTION
VANDAZOLE (metronidazole gel, USP), 0.75% is the vaginal dosage form of the nitroimidazole antimicrobial metronidazole at a concentration of 0.75%. Chemically, metronidazole is a 2-methyl-5-nitroimidazole-1-ethanol.
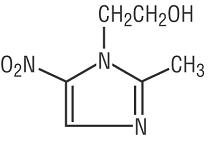
C6H9N3O3 M.W. 171.16
VANDAZOLE is a colorless to yellow gel, containing metronidazole, USP at a concentration of 7.5 mg/g (0.75%). The gel also contains edetate disodium, hypromellose, methylparaben, propylene glycol, propylparaben, purified water, and sodium hydroxide (to adjust pH).
Each applicator full of 5 grams of vaginal gel contains approximately 37.5 mg of metronidazole, USP.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Metronidazole is an antibacterial drug [see Clinical Pharmacology, Microbiology (12.4)]
12.3 Pharmacokinetics
Healthy Subjects
Following a single, intravaginal 5 gram dose of metronidazole vaginal gel (equivalent to 37.5 mg of metronidazole) to 38 healthy female subjects, a mean maximum serum metronidazole concentration of 281 ng/mL was reported (range: 134 to 464 ng/mL). The average time to achieve this Cmax was 9.5 hours (range: 4 to 17 hours) after dosing with metronidazole vaginal gel. This Cmax is approximately 2% of the mean maximum serum concentration reported in healthy subjects administered a single, oral 500 mg dose of metronidazole (mean Cmax = 12,785 ng/mL).
The extent of exposure [area under the curve (AUC)] of metronidazole, when administered as a single intravaginal 5 gram dose of metronidazole vaginal gel (equivalent to 37.5 mg of metronidazole), was 5,989 ng•hr/mL (range: 2,797 to 10,515 ng•hr/mL). This AUC0-∞ is approximately 5% of the reported AUC of metronidazole following a single oral 500 mg dose of metronidazole approximately 125,000 ng•hr/mL.
Patients with Bacterial Vaginosis
Following single and multiple 5 gram doses of a similar metronidazole vaginal gel product to 4 patients with bacterial vaginosis, a mean maximum serum metronidazole concentration of 214 ng/mL on Day 1 and 294 ng/mL (range: 228 to 349 ng/mL) on Day 5 were reported. Steady state metronidazole serum concentrations following oral dosages of 400 to 500 mg twice a day have been reported to range from 6,000 to 20,000 ng/mL.
12.4 Microbiology
Mechanism of Action
The intracellular targets of action of metronidazole on anaerobes are largely unknown. The 5-nitro group of metronidazole is reduced by metabolically active anaerobes, and studies have demonstrated that the reduced form of the drug interacts with bacterial DNA. However, it is not clear whether interaction with DNA alone is an important component in the bactericidal action of metronidazole on anaerobic organisms.
Activity In Vitro
Metronidazole is an antibacterial agent active in vitro against most strains of the following organisms that have been reported to be associated with bacterial vaginosis:
Bacteroides spp.
Gardnerella vaginalis
Mobiluncus spp.
Peptostreptococcus spp.
Standard methodology for the susceptibility testing of the potential bacterial vaginosis pathogens, Gardnerella vaginalis and Mobiluncus spp., has not been defined. Culture and sensitivity testing of bacteria are not routinely performed to establish the diagnosis of bacterial vaginosis [see Clinical Studies (14)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Metronidazole has shown evidence of carcinogenic activity after chronic oral administration in mice and rats. Pulmonary tumors and lymphomas were reported in several oral mouse studies in which mice were dosed at 75 mg/kg and above (about 5 times the clinical human dose based on body surface area comparison). Malignant liver tumors were reported in male mice dosed at doses equivalent to a human dose of 41 mg/kg/day (33 times the recommended clinical dose based on body surface area comparisons). Chronic oral dosing of metronidazole in rats at doses above 150 mg/kg (about 20 times the clinical human dose based on body surface area comparison) has resulted in mammary and hepatic tumors. Two lifetime tumorigenicity studies in hamsters have been performed and reported to be negative. Although no life-time studies were performed to evaluate the carcinogenic potential of VANDAZOLE (metronidazole gel, USP) 0.75%, published data have shown that intravaginal administration of metronidazole to Wistar rats for 5 days, at doses 26 times the recommended human dose based on body surface area comparisons, has resulted in an increased frequency of micronuclei in rat vaginal mucosal cells.
Metronidazole has shown mutagenic activity in a number of in vitro assay systems. In addition, a dose dependent increase in the frequency of micronuclei was observed in mice after intraperitoneal injections. An increase in chromosome aberrations has been reported in one study of patients with Crohn’s disease who were treated with 200 to 1200 mg/day of metronidazole for 1 to 24 months. However, in a second study, no increase in chromosome aberrations was reported in patients with Crohn’s disease who were treated with metronidazole for 8 months.
Fertility studies have been performed in mice up to six times the recommended human oral dose (based on mg/m2) and have revealed no evidence of impaired fertility. Metronidazole failed to produce any adverse effects on fertility or testicular function in male rats at doses up to 400 mg/kg/day (about 50 times the maximum recommended clinical dose based on body surface area comparisons) for 28 days. However, male rats treated at the same dose for 6 weeks or longer were infertile and showed severe degeneration of the seminiferous epithelium in the testes as well as marked decreases in testicular spermatid counts and epididymal sperm counts. Fertility was restored in most rats after an eight-week, drug-free recovery period.
14 CLINICAL STUDIES
A single, randomized, double-blind, active-controlled clinical trial was conducted to evaluate the efficacy of VANDAZOLE for the treatment of bacterial vaginosis. A clinical diagnosis of bacterial vaginosis was defined by the presence of a homogeneous vaginal discharge that (a) has a pH of greater than 4.5, (b) emits a "fishy" amine odor when mixed with a 10% KOH solution, and (c) contains clue cells on microscopic examination. Gram's stain results consistent with a diagnosis of bacterial vaginosis include (a) markedly reduced or absent Lactobacillus morphology, (b) predominance of Gardnerella morphotype, and (c) absent or few white blood cells. Non-pregnant females at least 18 years of age were randomized to receive treatment with either VANDAZOLE or another formulation of metronidazole vaginal gel 0.75% once daily at bedtime for 5 days. The modified intent-to treat population (patients who received study medication and had a Nugent score ≥ 4) consisted of 229 VANDAZOLE patients and 243 patients treated with another vaginal formulation of metronidazole. Therapeutic Cure defined as a clinical cure and Nugent score < 4 was assessed Day 22-31. Table 1 shows the therapeutic, clinical and Nugent score cure rates in this trial. The therapeutic cure rate was 42.8% for the VANDAZOLE group and 30.9% for the comparator group (95% confidence interval about the 11.9% difference in therapeutic cure rate: 2.8% to 21.0%).
|
Outcome |
Vandazole |
Active Control |
Treatment Difference (%) |
|
% Cure |
% Cure | ||
|
Therapeutic Cure |
42.8 |
30.9 |
11.9 [2.8, 21.0] |
|
Clinical Cure |
52.4 |
45.3 |
7.1 [-2.3, 16.5] |
|
Nugent Score Cure |
52.0 |
41.1 |
10.9 [1.5, 20.3] |
|
1 Modified intent-to-treat population |
|||
16 HOW SUPPLIED/STORAGE AND HANDLING
VANDAZOLE (metronidazole gel, USP), 0.75 % is supplied in a 70-gram tube and is packaged with 5 vaginal applicators. (NDC 0245-0860-70)
Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]. Avoid exposure to extreme heat or cold. Protect from freezing.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Interaction with Alcohol
Instruct the patient not to consume alcoholic beverages and preparations containing ethanol or propylene glycol during and for at least 3 days after treatment with VANDAZOLE [see Contraindications (4.3) and Drug Interactions (7.2)].
Drug Interactions
Instruct the patient not to use VANDAZOLE if disulfiram had been used within the last two weeks [see Contraindications (4.2)], and to inform their healthcare provider if they are taking oral anticoagulants, or lithium [see Drug Interactions (7.3, 7.4)].
Vaginal Intercourse and Use with Vaginal Products
Instruct the patient not to engage in vaginal intercourse or use other vaginal products (such as tampons or douches) during treatment with VANDAZOLE.
Fungal Vaginal Infections
Inform the patient that vaginal fungal infections can occur following use of VANDAZOLE and may require treatment with an antifungal drug [see Adverse Reactions (6.1)].
Lactation
A patient may choose to pump and discard breastmilk during treatment with VANDAZOLE and for 48 hours after last dose, and feed her infant previously stored human milk or formula [see Use in Specific Populations (8.2)].
Accidental Exposure to the Eye
Inform the patient that VANDAZOLE contains ingredients that may cause burning and irritation of the eye. In the event of accidental contact with the eye, rinse the eye with copious amounts of cool tap water and consult a healthcare provider.
Vaginal Irritation
Inform the patient to discontinue use and consult a healthcare provider if vaginal irritation occurs with use of VANDAZOLE.
Administration of Drug
Instruct the patient that VANDAZOLE (metronidazole gel, USP), 0.75% is supplied with 5 vaginal applicators. For once daily dosing, one applicator full should be used per dose.
US Patent No. 7,456,207
Manufactured In Croatia By:
Pliva Hrvatska d.o.o.
Zagreb, Croatia
Manufactured For:
UPSHER-SMITH LABORATORIES, LLC
Maple Grove, MN 55369
Rev. H 2/2021
PATIENT INSTRUCTIONS FOR USE
VANDAZOLE® (van-DA-zole)
(metronidazole gel, USP), 0.75%
For vaginal use only.
Do not put VANDAZOLE in your eyes, mouth, or on your skin.
Read this Patient Instructions for Use before you start using VANDAZOLE. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
Instructions for use:




1. Filling the applicator
- Remove the cap and puncture the metal seal on the tube with the pointed tip of the cap. (See Figure A)
- Screw the end of applicator onto the tube. (See Figure B)
- Slowly squeeze the gel out of tube and into the applicator. The plunger will stop when the applicator is full. (See Figure C)
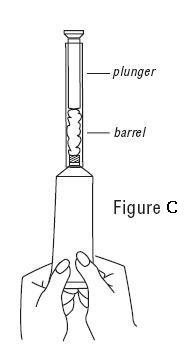
- Unscrew the applicator and replace the cap on the tube.
2. Inserting the applicator
- You can insert the applicator into your vagina:
- while you lie on your back with your knees bent or
- in any position that is comfortable for you
- Hold the filled applicator by the barrel, and gently insert into your vagina as far as it will comfortably go. (See Figure D)
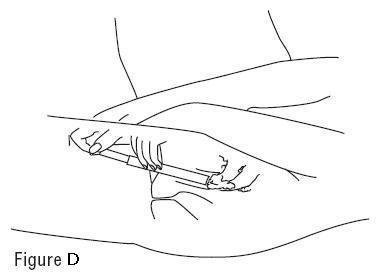
- Slowly push the plunger in until all of the gel goes into your vagina (See Figure D).
- Take the empty applicator out of your vagina.
3. Care of the applicator
This product comes with 5 vaginal applicators.
- After use, throw away the empty applicator in the trash.
While you use VANDAZOLE you should not have vaginal intercourse or use other vaginal products (such as tampons or douches).
If vaginal irritation develops when you use VANDAZOLE, discontinue use and consult your healthcare provider.
If you get VANDAZOLE in your eye, rinse your eye with cool tap water and consult a healthcare provider.
How should I store VANDAZOLE?
- Store VANDAZOLE at 68ºF to 77ºF (20ºC to 25ºC).
- Avoid exposure to extreme heat or cold. Avoid freezing VANDAZOLE.
- Keep this and all medicines out of reach of children.
Manufactured In Croatia By:
Pliva Hrvatska d.o.o.
Zagreb, Croatia
Manufactured For:
UPSHER-SMITH LABORATORIES, LLC
Maple Grove, MN 55369
© 2021 Upsher-Smith Laboratories, LLC
Rev. E 2/2021
