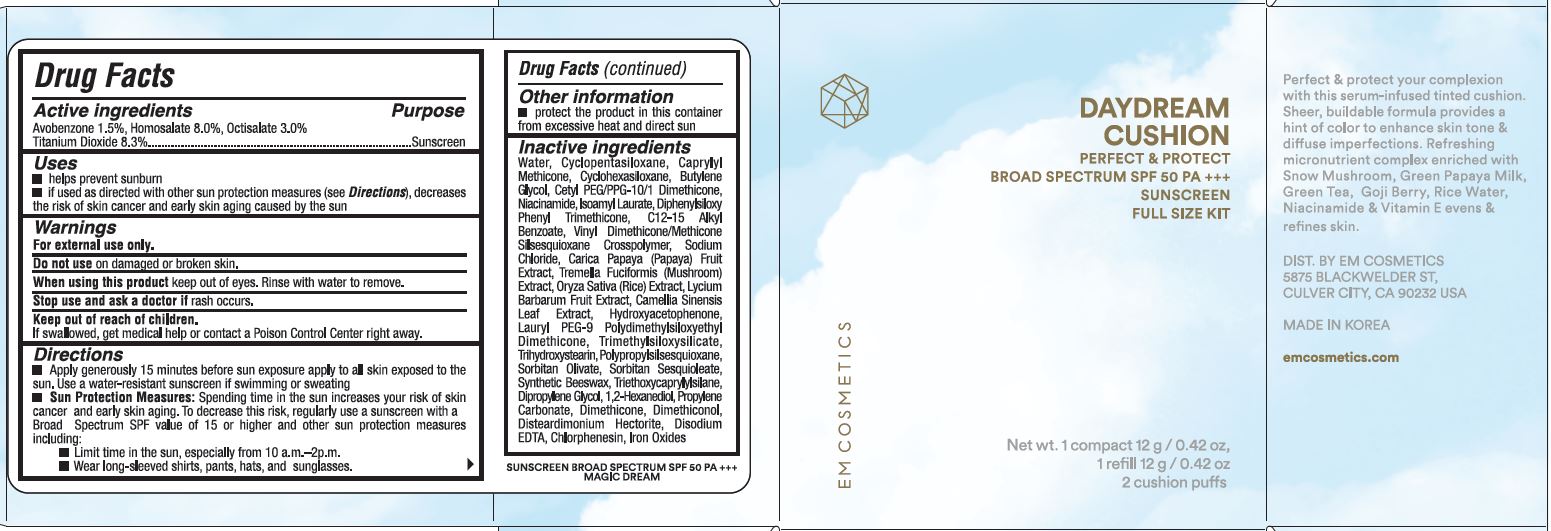
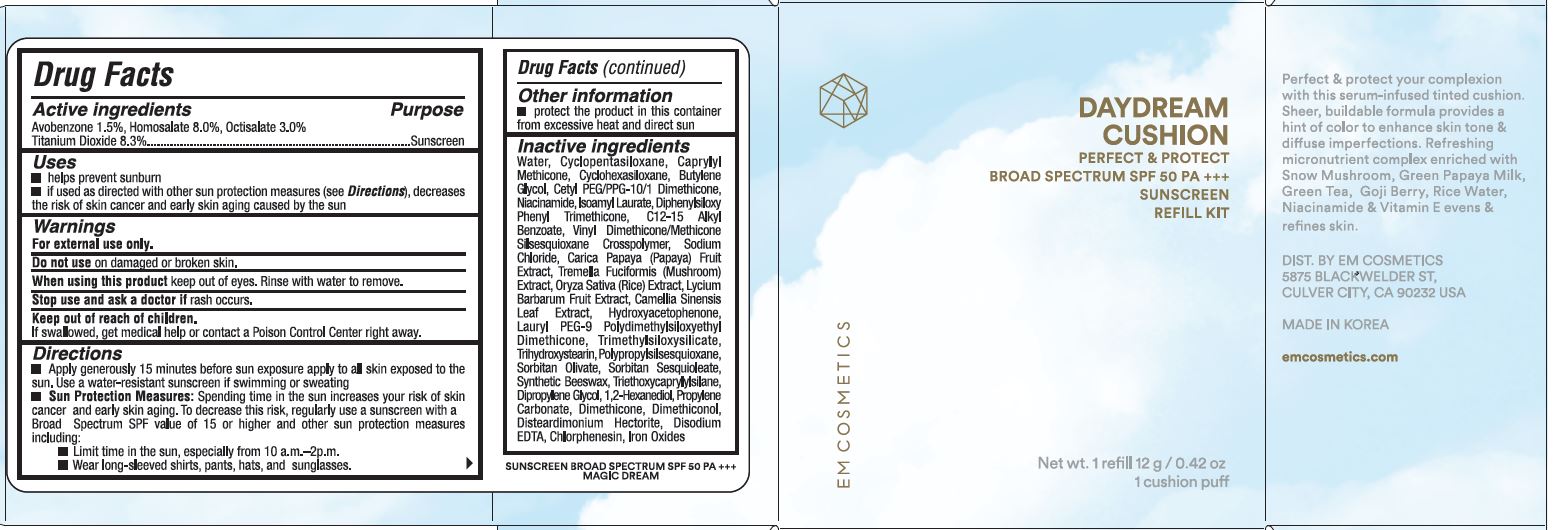
USES
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer, early skin aging by the sun.
WARNINGS
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
DIRECTIONS
- Apply generously 15 minutes before sun exposure apply to all skin exposed to the sun. Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer & early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
INACTIVE INGREDIENTS
WATER, CYCLOPENTASILOXANE, CAPRYLYL METHICONE, BUTYLENE GLYCOL, CETYL PEG/PPG-10/1 DIMETHICONE, CYCLOHEXASILOXANE, NIACINAMIDE, ISOAMYL LAURATE, DIPHENYLSILOXY PHENYL TRIMETHICONE, C12-15 ALKYL BENZOATE, VINYL DIMETHICONE/METHICONE SILSEQUIOXANE CROSSPOLYMER, SODIUM CHLORIDE, CARICA PAPAYA (PAPAYA) FRUIT EXTRACT, TREMELLA FUCIFORMIS (MUSHROOM) EXTRACT, ORYZA SATIVA (RICE) EXTRACT, LYCIUM BARBARUM FRUIT EXTRACT, CAMELIA SINENSIS LEAF EXTRACT, HYDROXYACETOPHENONE, TRIMETHYLSILOXYSILICATE, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, TRIHYDROXYSTEARIN, POLYPROPYLSILSESQUIOXANE, SORBITAN OLIVATE, SORBITAN SESQUIOLATE, SYNTHETIC BEESWAX, TRIETHOXYCAPRYLYLSILANE, DIPROPYLENE GLYCOL, 1,2-HEXANEDIOL, PROPYLENE CARBONATE, DIMETHICONE, DIMETHICONAL, DISTEARDIMONIUM HECTORITE, DISODIUM EDTA, CHLORPHENESIN, IRON OXIDES