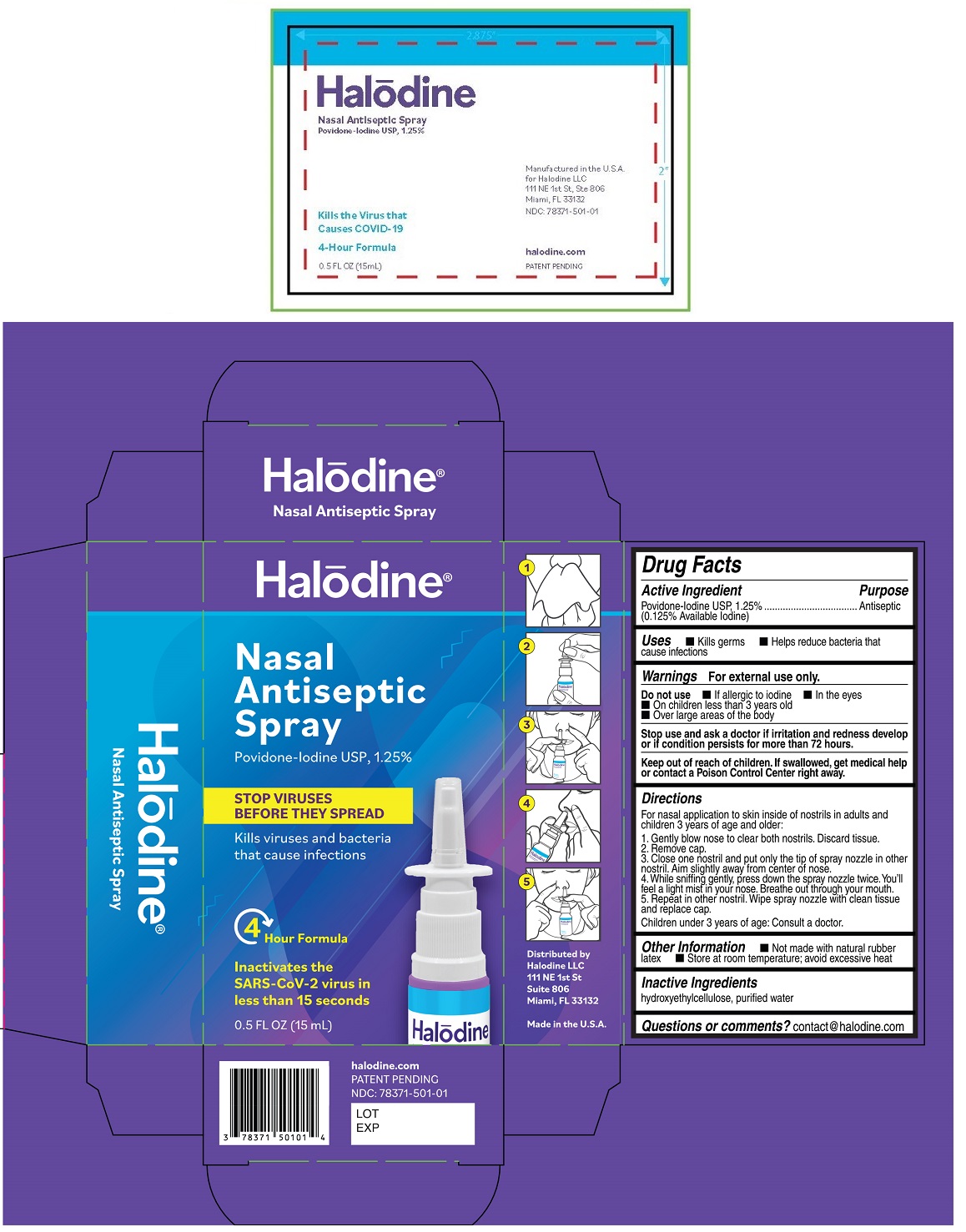
HALODINE NASAL ANTISEPTIC- povidone-iodine spray
Halodine Llc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Povidone-Iodine USP, 1.25%
(0.125% Available Iodine)
Uses
• Kills germs • Helps reduce bacteria that cause infections
Warnings
For external use only.
Do not use • If allergic to iodine • In the eyes
• On children less than 3 years old
• Over large areas of the body
Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For nasal application to skin inside of nostrils in adults and children 3 years of age and older:
1. Gently blow nose to clear both nostrils. Discard tissue.
2. Remove cap.
3. Close one nostril and put only the tip of spray nozzle in other nostril. Aim slightly away from center of nose.
4. While sniffing gently, press down the spray nozzle twice. You'll feel a light mist in your nose. Breathe out through your mouth.
5. Repeat in other nostril. Wipe spray nozzle with clean tissue and replace cap.
Children under 3 years of age: Consult a doctor.
Other Information
• Not made with natural rubber latex • Store at room temperature; avoid excessive heat
Inactive Ingredients
hydroxyethylcellulose, purified water
Questions or comments?
contact@halodine.com
STOP VIRUSES BEFORE THEY SPREAD
Kills viruses and bacteria that cause infections
Kills the virus that causes COVID-19
4 Hour Formula
Inactivates the SARS-CoV-2 virus in less than 15 seconds
Distributed by
Halodine LLC
111 NE 1st St
Suite 806
Miami, FL 33132
Made in the U.S.A
halodine.com
Packaging