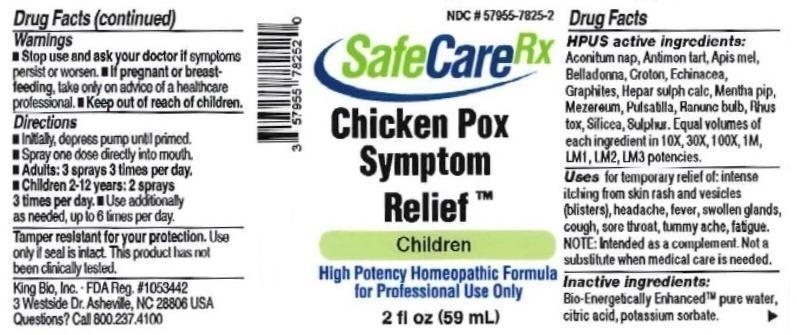
Drug Facts__________________________________________________________________________________________________________
HPUS active ingredients: Aconitum napellus, Antimonium tartaricum, Apis mellifica, Balladonna, Croton tiglium, Echinacea, Graphites, Hepar sulphuris calcareum, Mentha piperita, Mezereum, Pulsatilla, Ranunculus bulbosus, Rhus toxicodendron, Silicea, Sulphur. Equal volumes of each ingredient in 10X, 30X, 100X, 1M, LM1, LM2, LM3 potencies.
Uses temporary relief of: intense itching from skin rash and vesicles (blisters), headache, fever, swollen glands, cough, sore throat, tummy ache, fatigue. Note: Intended as a complement. Not a substitute when medical care is needed.
Warnings
- Stop use and ask your doctor if symptoms persist or worsen.
- If pregnant or breast-feeding, ask a healthcare professional before ues.
Directions
- Initially, depress pump until primed.
- Spray one dose directly into mouth.
- Ages 12 and up: 3 sprays 3 times per day.
- Children 2-12: 2 sprays 3 times per day.
- Use additionally as needed, up to 6 times per day.
Tamper resistant for your protection. Use only if safety seal is intact. This product has not been clinically tested.
Note: Intended as a complement. Not a substitute when medical care is needed.