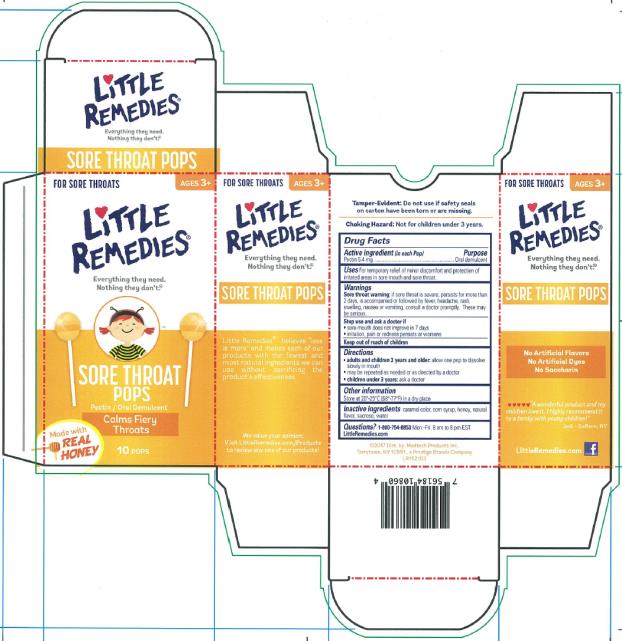
LITTLE REMEDIES SORE THROAT POPS- pectin lozenge
Medtech Products Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each Pop)
Pectin 5.4 mg
Uses
For temporary relief of minor discomfort and protection of irritated areas in sore mouth and sore throat.
Warnings
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea or vomiting, consult a doctor promptly. These may be serious.
Stop use and ask a doctor if
• sore mouth does not improve in 7 days
• irritation, pain or redness persists or worsens
Keep out of reach of children
Directions
• adults and children 3 years of age and older: allow one pop to dissolve slowly in mouth
• May be repeated as needed or as directed by a doctor
• Children under 3 years of age: ask a doctor
Other information
Store at 20°-25°C (68°-77°F) in a dry place
Inactive ingredients
caramel color, corn syrup, honey, natural flavor, sucrose, water
Questions? 1-800-754-8853
Mon.-Fri. 8 am to 8 pm EST
LittleRemedies.com
PRINCIPAL DISPLAY PANEL
Little Remedies
Sore Throat Pops
Pectin /Oral Demulcent
10 Pops
Medtech Products Inc.
