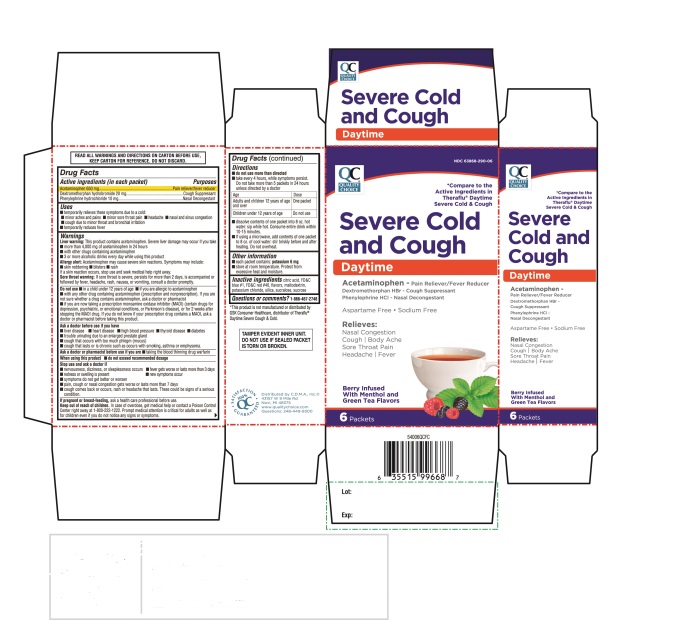
Active ingredients (in each packet)
Acetaminophen 650 mg
Dextromethorphan hydrobromide 20 mg
Phenylephrine hydrochloride 10 mg
Uses
- ▪
- temporarily relieves these symptoms due to a cold:
- ▪
- minor aches and pains
- ▪
- minor sore throat pain
- ▪
- headache
- ▪
- nasal and sinus congestion
- ▪
- cough due to minor throat and bronchial irritation
- ▪
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- ▪
- more than 4,000 mg of acetaminophen in 24 hours
- ▪
- with other drugs containing acetaminophen
- ▪
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- ▪
- skin reddening
- ▪
- blisters
- ▪
- rash
If a skin reaction occurs, stop use and seek medical help right away
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting consult a doctor promptly.
Do not use
- ▪
- in a child under 12 years of age
- ▪
- if you are allergic to acetaminophen
- ▪
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or a pharmacist.
- ▪
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask doctor before use if you have
- ▪
- liver disease
- ▪
- heart disease
- ▪
- high blood pressure
- ▪
- thyroid disease
- ▪
- diabetes
- ▪
- trouble urinating due to an enlarged prostate gland
- ▪
- cough that occurs with too much phlegm (mucus)
- ▪
- cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
Stop use and ask a doctor if
- ▪
- nervousness, dizziness, or sleeplessness occurs
- ▪
- fever gets worse or lasts more than 3 days
- ▪
- redness or swelling is present
- ▪
- new symptoms occur
- ▪
- symptoms do not get better or worsen
- ▪
- pain, cough or nasal congestion gets worse or lasts more than 7 days
- ▪
- cough comes back or occurs fever, rash or headache that lasts. These could be signs of a serious condition.
Directions
- ▪
- do not use more than directed
- ▪
- take every 4 hours; while symptoms persist. Do not to exceed 5 packets in 24 hours unless directed by a doctor
|
Age |
Dose |
|
Adults and children 12 years of age and over |
One packet |
|
Children under 12 years of age |
Do not use |
- •
- dissolve contents of one packet into 8 oz hot water; sip while hot. Consume entire drink within 10-15 minutes
- •
- If using a microwave, add contents of one packet 8 oz. of cool water: Stir briskly before and after heating: Do not overheat.
Other information
- ▪
- each packet contains: potassium 6mg
- ▪
- store at room temperature. Protect from excessive heat and moisture.
Inactive ingredients
citric acid, FD &C blue # 1, FD & C red # 40,flavors, maltodextrin, potassium chloride, silica ,sucralose, sucrose
Principal Display
QC Quality Choice
*Compare to the Active Ingredients in Theraflu® Daytime Severe Cold &Cough
SEVERE COLD & COUGH
Daytime
Acetaminophen
Pain Reliever/ Fever Reducer
Dextromethorphan HBr
Cough suppressant
Phenylephrine HCl
Nasal Decongestant
Aspartame Free ●Sodium Free
Relieves:
- Nasal Congestion
- Cough/Body Ache
- Sore Throat Pain
- Headache/Fever
Berry Infused with Menthol & Green Tea Flavors
6 Packets
|
READ ALL WARNINGS AND DIRECTIONS ON CARTON BEFORE USE, KEEP CARTON FOR REFERENCE, DO NOT DISCARD, |
*This product is not manufactured or distributed by GSK Consumer Healthcare, distributor of Theraflu® Daytime Severe Cough & Cold
TAMPER EVIDENT INNER UNIT: DO NOT USE IF SEALED PACKET IS TORN OR BROKEN.
Distributed by: C.D.M.A., Inc
43157 W. 9 Mile Rd
Novi, MI 48375
Questions: 248-449-9300