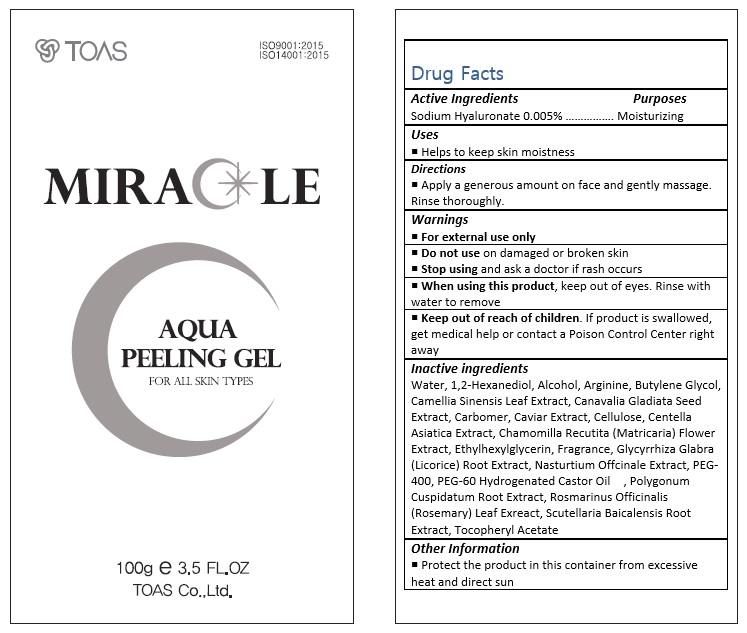
For external use only.
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove.
Stop using and ask a doctor if rash occurs.
Keep out of reach of the children. If product is swallowed, get medical help or contact a poison control center right away.
Water, 1,2-Hexanediol, Alcohol, Arginine, Butylene Glycol, Camellia Sinensis Leaf Extract, Canavalia Gladiata Seed Extract, Carbomer, Caviar Extract, Cellulose, Centella Asiatica Extract, Chamomilla Recutita (Matricaria) Flower Extract, Ethylhexylglycerin, Fragrance, Glycyrrhiza Glabra (Licorice) Root Extract, Nasturtium Offcinale Extract, PEG-400, PEG-60 Hydrogenated Castor Oil , Polygonum Cuspidatum Root Extract, Rosmarinus Officinalis (Rosemary) Leaf Exreact, Scutellaria Baicalensis Root Extract, Tocopheryl Acetate