PRINCIPAL DISPLAY PANEL
NDC 70771-1319-1
Dexmedetomidine
Hydrochloride Injection
200 mcg/2 mL
(100 mcg/mL)
For Intravenous Infusion Use
MUST BE DILUTED
2 mL Single-Dose Vial
Rx only
Zydus Pharmaceuticals

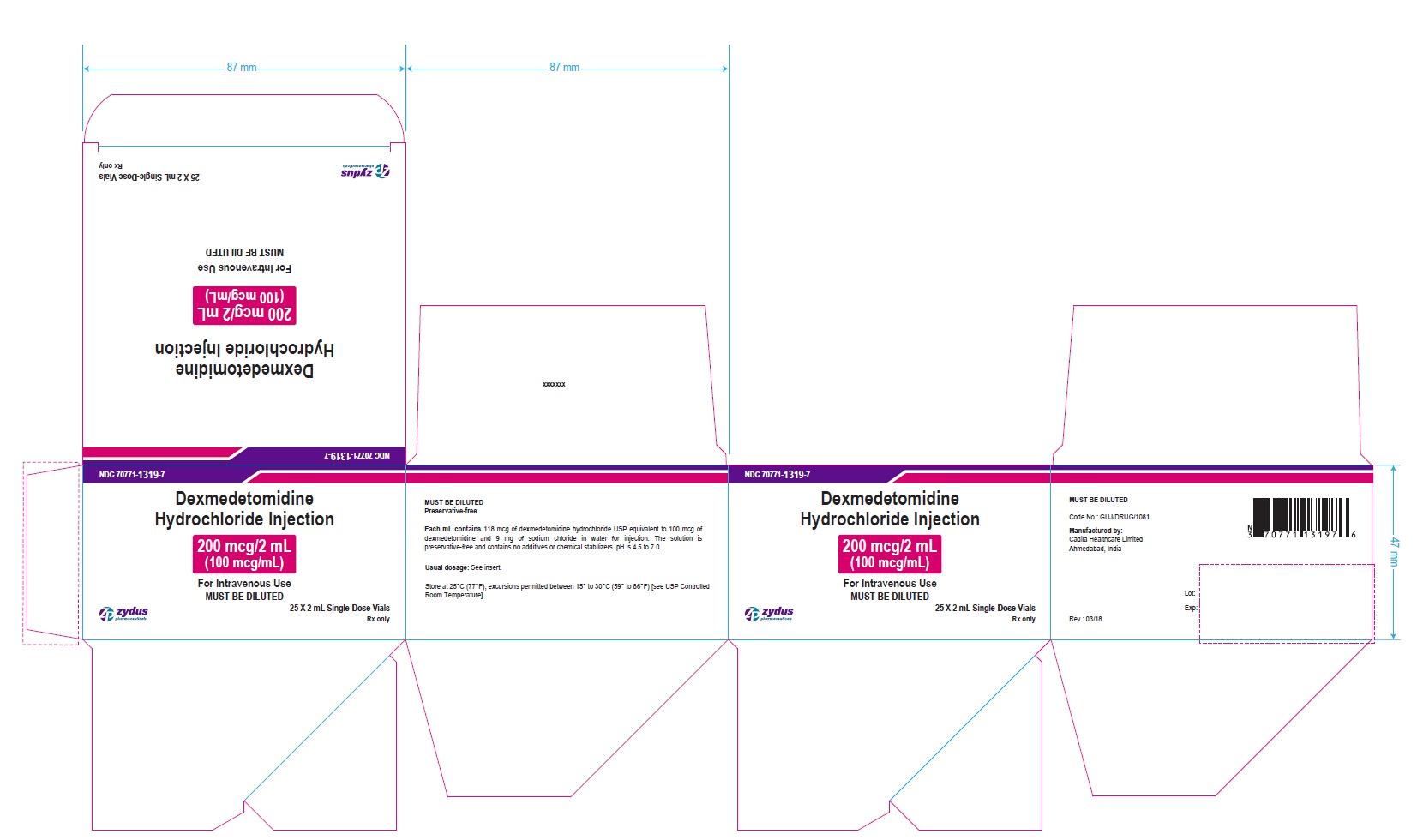
PRINCIPAL DISPLAY PANEL - 200 MCG/2 ML CARTON LABEL
NDC 70771-1319-7
Dexmedetomidine
Hydrochloride Injection
200 mcg/2 mL
(100 mcg/mL)
For Intravenous Infusion Use
MUST BE DILUTED
25 X 2 mL Single-Dose Vials
Rx only
Zydus Pharmaceuticals