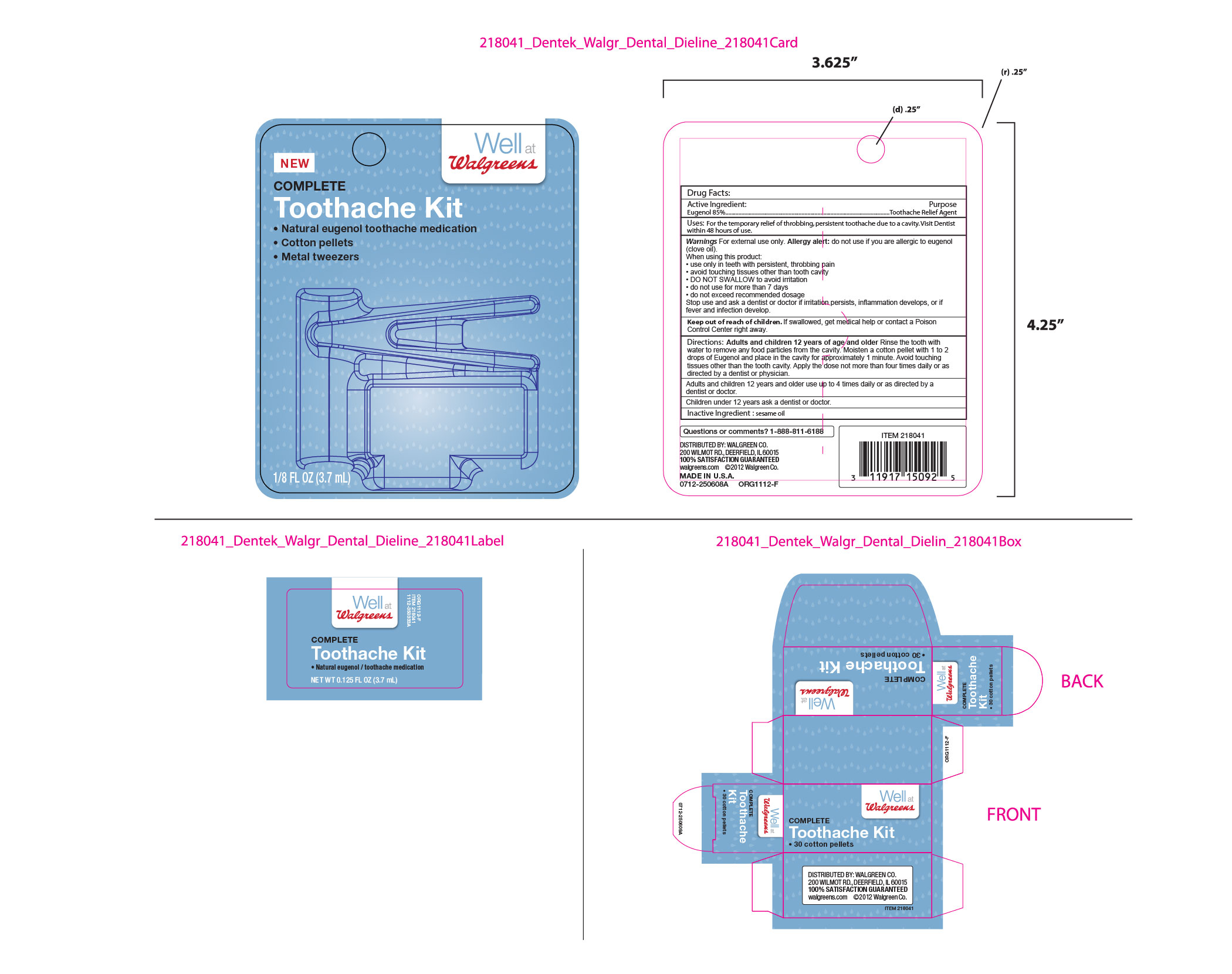
Uses: For the temporary relief of throbbing persistent toothache due to a cavity. Visit Dentist within 48 hours of use.
Warnings For external use only. Allergy alert: do not use if you are allergic to eugenol (clove oil).
When using this product:
. use only in teeth with pesistent, throbbing pain
. avoid touching tissues other than tooth cavity
. DO NOT SWALLOW to avoid irritation
. do not use for more than 7 days
. do not exceed recommended dosage
Stop use and ask a dentist or doctor if irritation persists, inflammation develops, or if fever and infection develop.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions:Adults and children 12 years of age and older Rinse the tooth with water to remove any food particles from the cavity.
Moisten a cotton pellet with 1 to 2 drops of Eugenol and place in the cavity for approximately 1 minute. Avoid touching tissues other than the tooth cavity. Apply the dose not more than four times daily or as directed by a dentist or physician.
Children under 12 years ask a dentist or doctor.