Uses
Use(s)
Enter section text here
Uses relieves
• heartburn
• acid indigestion
• sour stomach
• gas associated with these conditions
Warnings
Ask a doctor before use if you have
• kidney disease
• a magnesium restricted diet
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if syptoms last more than 2 weeks.
KEEP OUT OF REACH OF CHILDREN.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Directions
Shake well before use. Do not take more than 24 teaspoonful in 24 hours or use the maximum dosage for more than 2 weeks. Adults and children 12 years and older: take 2 to 4 teaspoonsfuls between meals, at bedtime, or as directed by a doctor.
Children under 12 years: ask a doctor.
Other Information
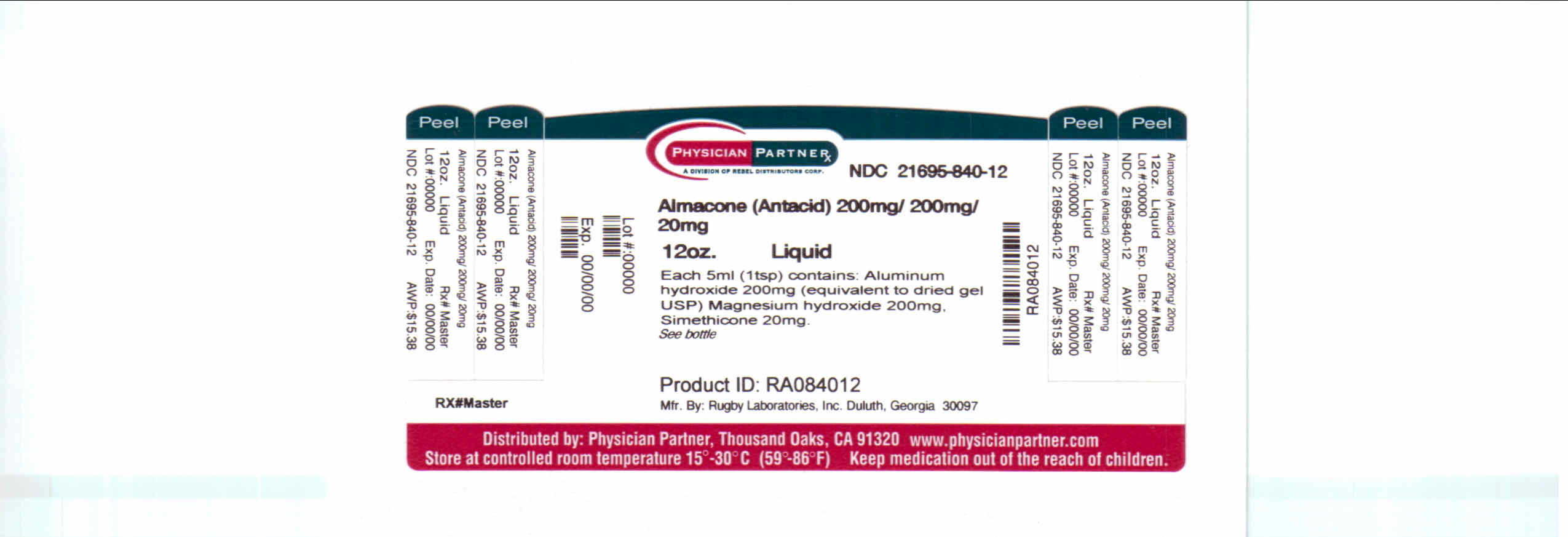
Each 5 mL teaspoon contains:
magnesium 25 mg, sodium 1 mg. Store at room temperature. Protect from freezing. Keep tighly closed.
Inactive ingredients
Benzoyl alcohol, butylparaben, carboxy methylcellulose sodium, flavor, hypromelloses, microcrystalline cellulose, propylparaben, purified water, saccharin sodium, sorbitol solution.