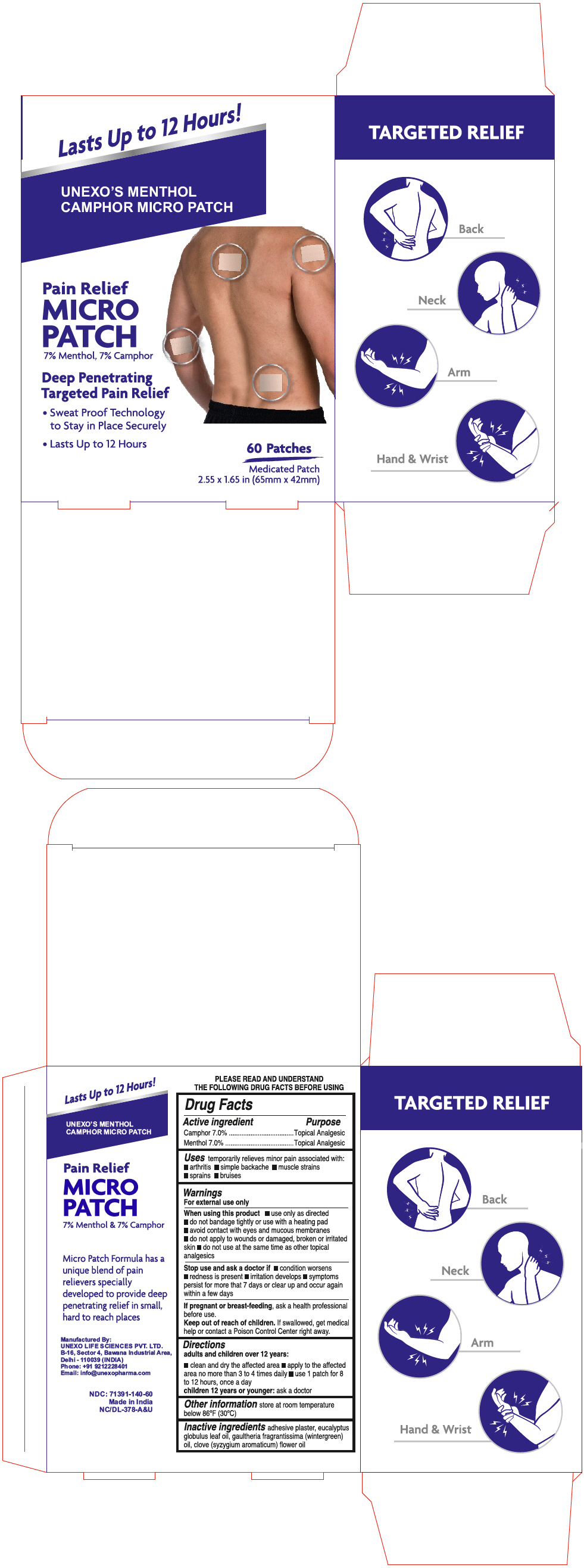
Uses
temporarily relieves minor pain associated with:
- Arthritis
- Simple backache
- Muscle strains
- Sprains
- Bruises
Warnings
For external use only
When using this product
- use only as directed
- Do not bandage tightly or use with a heating pad
- Avoid contact with eyes and mucous membranes
- Do not apply to wounds or damaged, broken or irritated skin
- Do not use at the same time as other topical analgesics
Directions
Adults and children 12 years:
- clean and dry the affected area
- apply to the affected area no more than 3 to 4 times daily
- Use 1 patch for 8 to 12 hours, once per day
Children under 12 years of age:
- Ask a doctor
Inactive Ingredients
Adhesive plaster, eucalyptus globulus leaf oil, gaultheria fragrantissima (wintergreen) oil, clove (syzygium aromaticum) flower oil
Manufactured By:
UNEXO LIFE SCIENCES PVT. LTD.
B-16. Sector 4, Bawana Industrial Area,
Delhi – 110039 (INDIA)
PRINCIPAL DISPLAY PANEL - 60 Patch Pouch Box
Lasts Up to 12 Hours!
UNEXO'S MENTHOL
CAMPHOR MICRO PATCH
Pain Relief
MICRO
PATCH
7% Menthol, 7% Camphor
Deep Penetrating
Targeted Pain Relief
- Sweat Proof Technology
to Stay in Place Securely - Lasts Up to 12 Hours
60 Patches
Medicated Patch
2.55 x 1.65 in (65mm x 42mm)