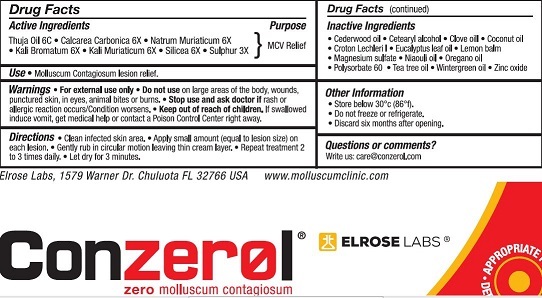
ACTIVE INGREDIENTS
SILICEA
CALCAREA CARBONICA
THUJA OIL
NATRUM MURIATICUM
KALI BROMATUM
SULPHUR
KALI MURIATICUM
CROTON LECHLERI RESIN
EMULSIFYING WAX NF
POLYSORBATE 60
MAGNESIUM SULFATE, UNSPECIFIED
VEGETABLE GLYCERIN
ZINC OXIDE
DIMETHYL ISOSORBIDE
TEA TREE OIL
METHYL SALICYLATE
CLOVE OIL
MELISSA OFFICINALIS
OREGANO LEAF OIL
CEDRUS ATLANTICA BARK OIL
NIAOULI OIL
EUCALYPTUS CAMALDULENSIS LEAF OIL
COCONUT OIL
DIRECTIONS
Clean infected skin area'.
Apply small amount (equal to lesion size) on each lesion.
Gently rub in circular motion leaving thin cream layer.
Repeat treatment 2 to 3 times daily.
Let dry for 3 minutes.