Directions
- swallow one or two softgels as symptoms occur
- do not exceed two softgels per 24 hours except under the advice and supervision of a physician
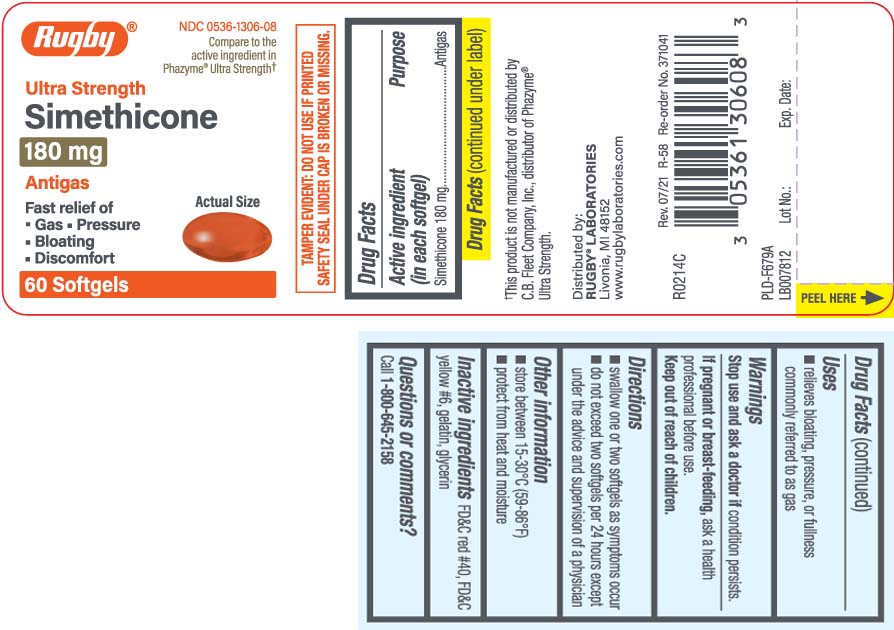
Principal Display Panel
Compare to the active ingredient in Phazyme® Ultra Strength†
Ultra Strength
Simethicone 180 mg
Antigas
Fast relief of
- Gas
- Pressure
- Bloating
- Discomfort
†This product is not manufactured or distributed by C.B. Fleet Company Inc., distributor of Phazyme® Ultra Strength
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Distributed by:
RUGBY® LABORATORIES
Livonia, MI 48152
.