FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
LANTIDRA is an allogeneic pancreatic islet cellular therapy indicated for the treatment of adults with Type 1 diabetes who are unable to approach target HbA1c because of current repeated episodes of severe hypoglycemia despite intensive diabetes management and education. Use LANTIDRA in conjunction with concomitant immunosuppression.
Limitations of Use
When considering the risks associated with the infusion procedure and long-term immunosuppression, there is no evidence to show a benefit of administration of LANTIDRA in patients whose diabetes is well-controlled with insulin therapy or patients with hypoglycemic unawareness who are able to prevent current repeated severe hypoglycemic events (neuroglycopenia requiring active intervention from a third party) using intensive diabetes management (including insulin, devices, and education).
Repeated intraportal islet infusions are not recommended in patients who have experienced prior portal thrombosis, unless the thrombosis was limited to second- or third-order portal vein branches.
There is no evidence to support the safe and effective use of LANTIDRA in patients with liver disease, renal failure, or who have received a renal transplant.
2 DOSAGE AND ADMINISTRATION
For infusion into the hepatic portal vein only.
2.1 Dose
The recommended minimum dose is 5,000 EIN/kg for initial infusion and 4,500 EIN/kg for subsequent infusion in the same recipient. The maximum dose per infusion is dictated by the estimated tissue volume, which should not exceed 10 cc per infusion, and the total EIN present in the infusion bag (up to a maximum of 1 × 106 EIN per bag).
A second infusion may be performed if the patient does not achieve independence from exogenous insulin within one year of infusion or within one year after losing independence from exogenous insulin after a previous infusion. A third infusion may be performed using the same criteria as for the second infusion. There are no data regarding the effectiveness or safety for patients receiving more than three infusions.
Pre-procedural medications
Provide pre-procedural induction immunosuppression 30 – 360 minutes prior to LANTIDRA infusion. Include the following, at the discretion of the treating physician who is experienced with management of immunosuppression regimens for islet cell transplantation:
- Non-depleting monoclonal anti-interleukin-2 (anti-IL-2) receptor antibody 120 minutes prior to islet infusion
- Note: In patients who are sensitized (hypersensitivity with a past history of anaphylactic reaction) to non-depleting monoclonal anti-interleukin-2 (anti-IL-2) receptor antibody therapies, a polyclonal, T-cell-depleting antibody should be used instead.
- Calcineurin inhibitor
- Mammalian target of rapamycin (mTOR) inhibitor
- Tumor necrosis factor (TNF) blocker.
- Periprocedural antibiotic prophylaxis is recommended.
2.2 Preparation
- Keep LANTIDRA in the insulated container at 15°C to 25°C no longer than 6 hours from time of product release (See carton label and certificate of analysis). Dispose of any product not used within 6 hours.
- Do not irradiate.
- Select and prepare units under the direction of a medical professional who is experienced in islet infusion (transplantation).
- Use LANTIDRA as supplied and without further dilution.
2.3 Administration
Failure to follow these directions may result in damage and decreased viability of the islets.
Do not administer with leukodepleting filters.
- To optimize viability, administer LANTIDRA as soon as possible after product release.
- Interventional radiologists and surgeons with expertise in islet cell infusion may administer LANTIDRA in an interventional radiology suite or operating suite under controlled aseptic conditions.
- Perform all steps aseptically.
- Use a 5 or 6 French angiographic catheter indicated for the delivery of drugs or other therapeutic fluids for infusion of LANTIDRA.
- Catheter length: 65 cm or less.
- Internal diameter: 0.97mm (0.038 inches) or greater.
- Use only sheaths and introducers in combination with a catheter with the specified dimensions listed above to deliver LANTIDRA.
Pre-Infusion Patient Preparation
- 1.
- Confirm the identity of the patient for the specified unit of LANTIDRA.
- 2.
- Confirm that the patient has received appropriate premedication [See Pre-procedural medication (2.1)].
- 3.
- Confirm that appropriate medications and blood products are available to manage any potential emergencies, such as hemorrhage, portal vein thrombosis, allergic reactions, glycemic lability, bleeding, and pain.
- 4.
- Confirm that the patient is hydrated adequately prior to infusion.
- 5.
- If indicated, administer a saline/glucose infusion and administer insulin using an intravenous insulin pump during the periprocedural period.
Pre-Infusion LANTIDRA Preparation
- 6.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- a.
- LANTIDRA is a cellular suspension (light yellow liquid with the presence of visible cellular aggregates).
- b.
- The Rinse Bag contains transplant media (light yellow liquid only with no cellular aggregates present).
- 7.
- Inspect the LANTIDRA infusion bag and the Rinse Bag for leaks and breaches of container integrity.
- 8.
- Ensure the connector between the LANTIDRA infusion bag and the Rinse Bag is secure and closed.
Note: If there are any product irregularities present or if the container appears damaged or otherwise compromised, do not infuse product and immediately notify the transplant physician/team and CellTrans at 1-800-500-1617
- 9.
- Gently agitate the LANTIDRA infusion bag to ensure that the islets are suspended and to prevent clumping. Do not shake the bag, as this may damage the islets. Repeat gentle agitation periodically throughout the infusion process.
- 10.
- Remove the first drape bag and transfer the product to an infusion operator to remove the second drape bag.
- 11.
- Ensure that the intravenous tubing is closed, then connect the LANTIDRA infusion bag, fill the drip chamber, and open the roller clamp to fill the tubing and remove air.
LANTIDRA Infusion Procedure
- 12.
- Insert the catheter into the portal vein.
- 13.
- Once the catheter placement in the portal vein is confirmed, connect the intravenous tubing from the LANTIDRA infusion bag to the catheter using a Luer lock connector.
- 14.
- Infuse all infusion bags by gravity flow over approximately 30 minutes at rates ≤ 25 mL/kg/h.
- 15.
- Flush the infusion lines periodically to clear them.
- 16.
- Do not administer LANTIDRA (islet cell product and rinse bag) through intravenous lines that contain any other medications or infusates other than physiological saline.
- 17.
- Reduce infusion rate if the fluid load is not tolerated.
- 18.
- Discontinue the infusion in the event of an allergic reaction or if the patient develops a moderate to severe infusion reaction.
- 19.
- Once the islet infusion is complete, open the roller clamp on the Rinse Bag tubing to allow refilling and rinsing of the LANTIDRA infusion bag. Gently agitate the LANTIDRA infusion bag with small amounts of rinse solution to ensure that all cells have been administered. Repeat until the Rinse Bag is empty.
- 20.
- Withdraw the catheter tip from the main portal vein into the liver parenchyma until it lies within a few centimeters (cm) of the liver capsule. Before withdrawing the catheter completely, manage hemostasis in the catheter track using standard practices to reduce the risk of bleeding.
Monitoring during LANTIDRA Infusion
- Measure portal pressure during the infusion.
- Pause infusion if portal pressure rises above 22 mmHg and do not resume until it falls below 18 mmHg.
- Terminate infusion if portal pressure remains above 22 mmHg for longer than 10 minutes.
- Monitor blood glucose levels every 15 minutes during the infusion and then every 30 minutes for the first 4 to 8 hours after infusion. Provide appropriate treatment if blood glucose levels fall below 70 mg/dL. Monitor blood glucose levels as needed once blood glucose levels have stabilized. After the acute period (first 4 to 8 hours following infusion), continue to monitor blood glucose (laboratory, capillary blood glucose, or continuous glucose monitor). Only use blood glucose meters and continuous glucose monitoring systems labelled for use in the hospital.
- Monitor the patient for portal vein branch thrombosis. Early diagnosis and prompt management with systemic heparinization may prevent clot propagation. However, anticoagulation therapy may lead to intra-abdominal hemorrhage requiring blood transfusion and surgical intervention.
Post-Infusion
- Monitor the patient in hospital for a minimum of 24 hours.
- Perform an abdominal ultrasound and Doppler examination of the liver after catheter removal to detect portal vein thrombosis and intra-abdominal bleeding. Repeat these examinations at least on days 1 and 7 post infusion procedure.
- Continue to monitor the patient for adverse reactions.
- Continue to monitor blood glucose levels following infusion and manage according to inpatient standard of care.
Post-Infusion Medications
- Anti-infective medications: Administer Pneumocystis jirovecii pneumonia (PCP) and cytomegalovirus (CMV) prophylaxis immediately following infusion of LANTIDRA and continue treatment as described in the prescribing information for the specific anti-infective medications.
- A non-depleting monoclonal anti-IL-2 receptor antibody: Administer at Week 2 after infusion for a total of two (2) doses, except in sensitized patients, who should instead be administered a polyclonal, T-cell-depleting antibody.
- Tumor necrosis factor (TNF) blocker: Administer on post-infusion Days 3, 7, and 10.
Long-term Medications
Immunosuppression: Continue immunosuppression permanently to prevent islet graft rejection. [See Warnings and Precautions (5.1)]. (See Section 5.1 for reasons to discontinue immunosuppression.)
Avoid systemic steroids. Use a combination of a calcineurin inhibitor and an mTOR inhibitor or appropriate alternatives, at the discretion of the physician. Monitor trough levels of maintenance immunosuppressant drugs, and adjust the dose to maintain appropriate blood levels.
3 DOSAGE FORMS AND STRENGTHS
LANTIDRA is a cellular suspension of allogeneic pancreatic islets (islets of Langerhans) in buffered transplant media containing sodium chloride, dextrose, minerals, amino acids, vitamins, and other compounds supplemented with HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid; 10 mM final concentration) and human serum albumin (0.5% final concentration).
Each infusion uses one lot of LANTIDRA which consists of islets manufactured from the pancreas of a single deceased donor. Each dose of LANTIDRA is provided as two (2) infusion bags connected to each other via sterile connector. One bag contains LANTIDRA up to a maximum of 1 × 106 EIN in 400 ml of transplant media and the second bag (Rinse Bag) contains transplant media used to rinse the LANTIDRA bag and the infusion line.
The dosage strength is represented by the total EIN in a single preparation and varies between product batches. Dosage strength information for an individual batch is provided on the container label and in accompanying documentation (Final Islet Product Certificate of Analysis).
4 CONTRAINDICATIONS
Do not administer LANTIDRA to patients who have concomitant diseases or conditions, including pregnancy, that contraindicate the procedure for LANTIDRA infusion or immunosuppression.
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Immunosuppression
Concomitant use of immunosuppression is required to maintain islet cell viability. The use of immunosuppression in patients receiving LANTIDRA increases the risk of serious and potentially fatal adverse reactions. [Adverse Reactions (6.1)]
Patients receiving immunosuppressants are at increased risk of:
- Bacterial, viral, fungal, and parasitic infections, including opportunistic infections.
- Lymphomas and other malignancies, particularly of the skin.
- Severe anemia, sometimes requiring transfusion.
Before Treatment
- Vaccination: To mitigate the risk of infection, patients should receive recommended immunizations prior to treatment.
After Treatment
- Administer PCP and CMV prophylaxis following administration of LANTIDRA.
- Avoid live vaccination while receiving immunosuppression.
- Monitor for fever and other signs of infection; initiate appropriate treatment early.
- Clinically monitor for malignancy, including skin cancer.
- Monitor hemoglobin/hematocrit and give blood products as indicated.
Considerations for discontinuation of immunosuppression
- If a patient develops a life-threatening infection or cancer and treatment requires discontinuation of immunosuppression.
- If a patient has been dependent on exogenous insulin for two years after their last infusion, then immunosuppression should be discontinued. However, the treatment team may consider continuation of immunosuppression if they determine that the patient has achieved target HbA1c without recurrent severe hypoglycemia in the presence of clinically relevant C-peptide, that provides a potential ongoing benefit that outweighs the risks of severe and potentially life-threatening effects of immunosuppression.
- If a patient becomes pregnant.
5.2 Procedural Complications
Liver laceration, hemorrhage and intra-abdominal bleeding have occurred with portal administration of LANTIDRA. Manage hemostasis in the catheter track using standard practices following infusion of LANTIDRA to reduce the risk of bleeding. Monitor for bleeding clinically and with laboratory assessments. Blood transfusions have been required.
Elevation in portal blood pressure has occurred during and following intraportal islet infusion [Adverse Reactions (6.1)]. Monitor portal pressure; pause infusion if portal pressure rises above 22 mmHg and do not resume until it falls below 18 mmHg. Terminate infusion if portal pressure remains above 22 mmHg for longer than 10 minutes. [Dosage and Administration (2.3)]
Portal vein branch thrombosis may occur following infusion of LANTIDRA. Repeated intraportal islet infusions are not recommended in patients who have experienced prior portal thrombosis unless the thrombosis was limited to second- or third-order portal vein branches. [Limitations of Use (2.1)]
5.3 Increased Risk of Islet Graft Rejection
Patients with a positive T- and B-cell crossmatch between recipient serum and donor lymphocytes may immediately reject the islet cells. The T- and B-cell crossmatch assay is binary. T- and B-cell both need to be negative.
6 ADVERSE REACTIONS
Ninety percent (90%) of subjects had at least one serious adverse reaction. The major causes were attributed to:
- Infusion procedure
- liver laceration/hematoma, hemorrhage, and intra-abdominal bleeding (13%)
- elevation of portal pressure (7%)
- Immunosuppression
- Infection (87%)
- Malignancy (37%)
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of LANTIDRA in subjects with type 1 diabetes and hypoglycemic unawareness was demonstrated in two clinical trials (Study 1, Study 2) involving a total of 30 subjects who received between one and three doses of LANTIDRA. Duration between first and second transplant was one month to 2.8 years and between second and third dose from 3 months to 7.8 years (See Figure 1). Because of the variable duration of follow-up, number of infusions, and interval between infusions, adverse reactions were reported for the total duration for which each subject was followed. [Clinical Studies (14)] Subjects were followed for 0.3 to 14.5 years (mean 3 ± 3.7 years) after the first infusion.
Serious reactions were reported in 27 (90%) of subjects. There were two (7%) deaths; one death from multi-organ failure with sepsis (1.6 years after the first infusion), and one from progressive confusion, global atrophy and micro-ischemic disease (9.7 years after the first infusion). Both subjects were using immunosuppression at the time of the event. Additionally, 8 (27%) subjects experienced at least one life-threatening adverse reaction and 26 (87%) subjects experienced at least one severe reaction before their last follow-up.
Immunosuppression-Related Adverse Reactions
Risks of common community-acquired infections and opportunistic infections increases with immunosuppression. In total, 211 infections were reported for 26 subjects; one was life-threatening, 22 reactions severe, and 115 events moderate in severity. Additionally, one subject died of multi-organ failure from sepsis in the second year after infusion.
Discontinuation of immunosuppression resulted in loss of islet cell function and if achieved insulin independence. This was described for 8 (27%) subjects.
Malignancy risk is known to increase with immunosuppression. In total, 16 adverse reactions of malignancy were reported in 11 subjects; three malignancies were life-threatening. The malignancies included 12 skin cancers, and one post-transplant lymphoproliferative disease, one breast cancer, and one thyroid cancer. Anemia was reported in 24 (80%) of subjects. Of the 90 adverse reactions reported, one reaction was life-threatening (Hgb <6.5gm/dL), 9 reactions were severe (<8-6.5 gm/dL), and 27 reactions were moderate in severity (<10-8 gm/dL).
Anemia was attributed to bleeding because of procedural complications as well as immunosuppression. Transfusion was required for severe and life-threatening reactions. Overall, five transfusions were administered to five subjects. Three transfusions were for procedural related complications and two were non-procedure related. Alterations in red blood cell turnover and transfusion can alter the accuracy of HbA1c measurements. Therefore, in addition to monitoring for the development of anemia as a result of immunosuppression or a result of a procedural complications, healthcare providers should consider the occurrence of anemia in the interpretation and use of HbA1c in the management of patients with type 1 diabetes who have received LANTIDRA.
Procedural Complications
Serious reactions related to the 56 infusion procedures included one life-threatening liver laceration, one intraabdominal hemorrhage, and two perihepatic hematomata resulting in prolonged hospitalization. Manage hemostasis in the catheter track using standard practices following infusion of LANTIDRA to reduce the risk of bleeding.
Elevation in portal blood pressure may occur following intraportal islet infusion but is usually temporary. During clinical trials with LANTIDRA, the median peak portal blood pressure increase from baseline was 3 mmHg (range -3 to 18 mmHg). Elevated portal pressures ≥ 22 mmHg were reported during procedures for two subjects requiring cessation of the procedure, and incomplete delivery of LANTIDRA for one subject. Monitor portal pressure and halt islet infusion if portal pressure rises above 22 mmHg.
Panel Reactive Antibodies
Of the 30 subjects who received LANTIDRA, 28 subjects had panel reactive antibody (PRA) data. Overall, 6 of 28 (21%) had a transition from baseline Class I PRA < 20% to ≥ 20% after infusion. These included 1 of 9 (11%) who received one infusion, 3 of 12 (25%) who received two infusions, and 2 of 7 (29%) who received three infusions.
| Adverse Reaction | % Subjects Any Severity | % Treated Subjects Severity ≥ Grade 3* |
|---|---|---|
| Common Terminology Criteria for Adverse Events (CTCAE) Version 5 | ||
| Grade 3: (Severe) Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living. | ||
| Grade 4: (Life-threatening) consequences; urgent intervention indicated. | ||
| Grade 5: Death related to the adverse event. | ||
| Nausea | 83 | 7 |
| Fatigue | 83 | 3 |
| Anemia | 80 | 27 |
| Diarrhea | 80 | 13 |
| Abdominal pain | 67 | 7 |
| Asthenia (loss of overall energy) | 67 | 7 |
| Headache | 67 | 3 |
| Hyponatremia (low levels of sodium) | 63 | 13 |
| Transaminases increased | 63 | 7 |
| Upper respiratory tract infection | 63 | 3 |
| Vomiting | 60 | 7 |
| Urinary tract infection | 53 | 10 |
| Hypoalbuminemia (low levels of albumin) | 47 | 3 |
| Low density lipoprotein increased | 43 | 37 |
| Myalgia (muscle pain) | 43 | 3 |
| Sinusitis | 40 | 7 |
| Chills | 40 | 3 |
| Hemoglobin decreased | 37 | 3 |
| Tinnitus | 30 | 3 |
| Decreased appetite | 27 | 3 |
| Hypertension | 23 | 7 |
| Pneumonia | 20 | 17 |
| Hypercholesterolemia (increased cholesterol) | 20 | 3 |
| Depression | 20 | 3 |
| Menstruation irregular | 20 | 3 |
Common adverse reactions (occurring in ≥20% but ≤ 90% of subjects) independent of severity observed between initial infusion and 1 year following final infusion include:
Blood and lymphatic system disorders: anemia, leukopenia
Cardiac disorders: palpitations
Ear and labyrinth disorders: ear pain, tinnitus
Eye disorders: eye pain, vision blurred
Gastrointestinal disorders: abdominal pain, diarrhea, dry mouth, mouth ulceration, nausea, stomatitis, vomiting
General disorders and administration site conditions: asthenia, chills, edema peripheral, fatigue, feeling cold, thirst
Hepatobiliary disorders: hepatic steatosis, hyperbilirubinemia
Infections and infestations: herpes zoster, pneumonia, sinusitis, upper respiratory tract infection, urinary tract infection
Injury, poisoning and procedural complications: contusion
Investigations: aspartate aminotransferase increased, blood bicarbonate decreased, blood cholesterol increased, hemoglobin decreased, low density lipoprotein increased, transaminases increased
Metabolism and nutrition disorders: abnormal loss of weight, anorexia and bulimia syndrome, appetite disorder, decreased appetite, hypercholesterolemia, hyperkalemia, hypoalbuminemia, hypocalcemia, hypomagnesemia, hyponatremia
Musculoskeletal and connective tissue disorders: arthralgia, muscle spasms, musculoskeletal stiffness, myalgia, pain in extremity
Neoplasms benign, malignant and unspecified (including cysts and polyps): thyroid neoplasm
Nervous system disorders: disturbance in attention, dizziness, headache, hypoesthesia, tremor
Psychiatric disorders: anhedonia, anxiety, depressed mood, depression, insomnia, nervousness
Renal and urinary disorders: hematuria, hypertonic bladder, nocturia, pollakiuria, urinary incontinence
Reproductive system and breast disorders: menstruation irregular
Respiratory, thoracic and mediastinal disorders: cough, dysphonia, dyspnea, nasal congestion, oropharyngeal pain, sinus disorder
Skin and subcutaneous tissue disorders: acne, dry skin, onychoclasis, pruritus, rash
Vascular disorders: hypertension
Less common adverse reactions (occurring in ≥5% but <20% of subjects) observed between initial infusion and 1 year following final infusion include:
Blood and lymphatic system disorders: increased tendency to bruise, lymphadenopathy, neutropenia, thrombocytopenia
Cardiac disorders: myocardial ischemia
Ear and labyrinth disorders: deafness, vertigo
Endocrine disorders: hypoglycemia, thyroid cyst
Eye disorders: cataract, conjunctival hemorrhage, eye edema, eye pruritus
Gastrointestinal disorders: Barrett's esophagus, bowel movement irregularity, colitis, constipation, dyspepsia, gastroesophageal reflux disease, oral pain, toothache
General disorders and administration site conditions: catheter site pain, chest pain, feeling of body temperature change, gait disturbance, influenza like illness, injection site extravasation, mucosal inflammation, pain, pyrexia
Hepatobiliary disorders: cholelithiasis
Immune system disorders: sensitization
Infections and infestations: bacterial vaginosis, cellulitis, cytomegalovirus infection, ear infection, Epstein-Barr infection, eye infection, fungal infection, gastroenteritis, gastroenteritis viral, localized infection, nail infection, nasopharyngitis, onychomycosis, oral candidiasis, oral herpes, osteomyelitis, rhinitis, tooth infection, vaginal infection, viral upper respiratory tract infection, vulvovaginal mycotic infection
Injury, poisoning and procedural complications: hepatic hematoma, limb injury, meniscus injury
Investigations: alanine aminotransferase increased, blood alkaline phosphatase increased, blood creatinine increased, glomerular filtration rate decreased, neutrophil count decreased, urine albumin/creatinine ratio, urine protein/creatinine ratio increased, weight decreased, weight increased
Metabolism and nutrition disorders: dehydration, hyperchloremia, hyperlipidemia, hypertriglyceridemia, hypokalemia, hypophosphatemia
Musculoskeletal and connective tissue disorders: arthritis, back pain, intervertebral disc protrusion, joint stiffness, joint swelling, muscular weakness, musculoskeletal pain, neck pain, osteoarthritis, osteopenia, osteoporosis
Neoplasms benign, malignant and unspecified (including cysts and polyps): basal cell carcinoma, squamous cell carcinoma
Nervous system disorders: carpal tunnel syndrome, cognitive disorder, dysgeusia, dyskinesia, head titubation, migraine, neuropathy peripheral, paresthesia, poor quality sleep, sinus headache, syncope
Psychiatric disorders: agitation, decreased interest, libido decreased
Renal and urinary disorders: hemoglobinuria, hydronephrosis, proteinuria, urine flow decreased
Reproductive system and breast disorders: erectile dysfunction, menorrhagia, vaginal hemorrhage
Respiratory, thoracic and mediastinal disorders: dyspnea exertional, epistaxis, pleural effusion, rhinorrhea, wheezing
Skin and subcutaneous tissue disorders: alopecia, dermatitis, erythema, hidradenitis, nail disorder, night sweats, rash pruritic, rosacea, skin exfoliation, skin lesion
Vascular disorders: peripheral artery stenosis
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Pregnancy risk has not been assessed for LANTIDRA. No animal reproductive and development toxicity studies have been conducted with LANTIDRA. However, there is a risk of fetal malformations associated with certain immunosuppression medications that may be used following LANTIDRA administration. Additionally, the risks to the patient and fetus from the procedure for LANTIDRA infusion in pregnant women has not been assessed.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
The risk of exposing a child to LANTIDRA components during breastfeeding has not been assessed. However, some required concomitant medications, including immunosuppressants, may be excreted in milk at least in trace amounts. Because of this, a decision should be made about whether to discontinue breastfeeding in patients who will receive a LANTIDRA infusion.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Due to the risk of fetal malformations associated with required concomitant medications, including immunosuppressants, females of reproductive potential should have a confirmed negative pregnancy test prior to LANTIDRA infusion.
Female patients of reproductive potential should be counselled to contact their transplant team immediately if they become pregnant.
Contraception
Because long-term immunosuppression is required following LANTIDRA administration, women of childbearing potential should be informed of the potential risks that these medications pose during pregnancy and should be told to use effective contraception prior to initiation of immunosuppression and thereafter for as long as they retain reproductive potential.
8.4 Pediatric Use
The safety and effectiveness of LANTIDRA have not been established in pediatric patients with type 1 diabetes.
8.5 Geriatric Use
The safety and effectiveness of LANTIDRA have not been established in geriatric patients with type 1 diabetes and hypoglycemic unawareness. Clinical studies of LANTIDRA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
11 DESCRIPTION
LANTIDRA consists of a suspension of allogeneic pancreatic islets in buffered transplant medium containing sodium chloride, dextrose, minerals, amino acids, vitamins, and other compounds supplemented with HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid; 10 mM final concentration) and human serum albumin (0.5% final concentration).
The active ingredient in LANTIDRA is allogeneic islets of Langerhans derived from a donor pancreas. Islets contain several types of endocrine (hormone-secreting) cells, including β-, α-, pancreatic peptide- (PP-), δ-, and ε-cells.
Each single-donor islet batch consists of two infusion bags connected to each other via a sterile connector. One LANTIDRA bag containing up to a maximum of 1 × 106 EIN in 400 ml of transplant media, and the second Rinse Bag containing 200 ml transplant media used to rinse the LANTIDRA bag and the infusion line.
Ingredients present in transplant media are:
CaC12, anhydrous, biotin, MgSO4, anhydrous, folic acid, Na acetate, anhydrous, riboflavin, NaH2PO4H2O, cocarboxylase, dextrose, Li3 coenzyme A 2 H2O, KCl, cozymase, NaCl, Na2 flavin adenine dinucleotide, Na gluconate H2O, Na triphosphopyridine nucleotide, L-alanine, Na3 uridine 5'-triphosphoric acid H2O, L-arginine HCl, ascorbic acid, L-aspartic acid, D-Ca-pantothenate, L-cysteine HCl H2O, choline chloride, L-cystine 2 HCl, i-inositol, L-glutamic acid, nicotinic acid, glycine, nicotinamide, L-histidine HCl H2O, para-aminobenzoic acid, hydroxy-L-proline, pyridoxine HCl, L-isoleucine, thiamine HCl, L-leucine, glutathione (reduced), L-lysine HCl, thymidine, L-methionine, 2D-adenosine, L-phenylalanine, 2D-cytidine HCl, L-proline, 2D-guanosine, L-serine, 5-methyl-2'- deoxycytidine, L-threonine, cholesterol, L-tryptophan, Tween 80, L-valine, L-alanyl-L-glutamine, L-tyrosine 2 Na 2 H2O
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pancreatic islets regulate blood glucose levels through secretion of multiple hormones in response to increases and decreases in blood glucose. Endocrine cells within pancreatic islets release insulin, glucagon, somatostatin, pancreatic peptide, and ghrelin. Insulin stimulates glucose uptake by peripheral tissues; glucagon mobilizes glucose from the liver into circulation; somatostatin inhibits both α- and β-cell secretions; pancreatic peptide inhibits pancreatic exocrine secretion; and ghrelin inhibits insulin secretion. The primary mechanism of action of LANTIDRA is believed to be secretion of insulin by infused (transplanted) β- cells.
12.2 Pharmacodynamics
The pharmacodynamic effects of LANTIDRA are a result of hormones, especially insulin, that are secreted by the infused (transplanted) islets in response to fluctuations in blood glucose levels.
Basal and stimulated blood glucose were determined at baseline and at 1 year following a subject's last transplant during Study 1 and Study 2 using a mixed meal tolerance test (MMTT). Combined results from these studies are summarized in Table 2. [Clinical Studies (14)]
The pharmacodynamic profile of the allogeneic islet cells is most clearly demonstrated in subjects who are free from the requirement of exogenous insulin.
| Subjects Insulin Independent at time of 1-year MMT | N | Mean (mg/dl) | Std Dev (mg/dl) | Min (mg/dl) | Max (mg/dl) |
|---|---|---|---|---|---|
| Baseline Glucose Basal | 19 | 178 | 76 | 78 | 348 |
| Baseline Glucose 90-min | 19 | 357 | 91 | 122 | 559 |
| 1-year Glucose Basal | 19 | 106 | 17 | 81 | 144 |
| 1-year Glucose 90-min | 19 | 142 | 40 | 65 | 202 |
14 CLINICAL STUDIES
The effectiveness of LANTIDRA in subjects with type 1 diabetes and hypoglycemic unawareness was demonstrated in 2 clinical trials (Study 1, Study 2) involving a combined 30 subjects, all of whom received at least one islet infusion and a maximum of 3 infusions. Both trials were prospective, open-label, single-arm studies.
Subject demographics: median age 46.5 (range: 21 – 67) years, 80% female, 100% white, 97% non-Hispanic.
Subjects received a median islet number of 399,178 EIN (range 253,924 EIN to 858,856 EIN) per infusion. Subjects received a median islet dose of 6,570 EIN/kg (range 4,186 EIN/kg to 13,633 EIN/kg) per infusion. Thirty subjects participated in the combined Study 1 and Study 2, with 11 subjects receiving one infusion, 12 subjects receiving two infusions, and 7 subjects receiving three infusions. Of the 19 subjects who received a second infusion, 6 were insulin-independent at the time of their second infusion. Of the 11 subjects who did not receive a second infusion, 4 were insulin-independent, 3 did not have a donor, and 4 were intolerant to immunosuppression or withdrew from the study within 6 months. All 7 subjects who received a third infusion were insulin-dependent. One subject was not able to get a third infusion because of infection. No subject was unable to receive a third infusion because of lack of a donor or intolerance to immunosuppression.
Concomitant study medications were provided as described in Table 3:
| Medication | Study 1 (N=10) | Study 2 (N=20) |
|---|---|---|
| Anakinra; n (%) | 1 (10%) | 0 (0%) |
| Daclizumab; n (%) | 10 (100%) | 5 (24%) |
| Basiliximab; n (%) | 5 (10%) | 19 (95%) |
| Mycophenolate mofetil; n (%) | 6 (60%) | 5 (24%) |
| Etanercept; n (%) | 6 (60%) | 20 (100%) |
| Everolimus; n (%) | 1 (10%) | 2 (10%) |
| Sirolimus; n (%) | 10 (100%) | 20 (100%) |
| Tacrolimus; n (%) | 10 (100%) | 20 (100%) |
| Cyclosporine; n (%) | 1 (10%) | 3 (15%) |
| Anti-thymocyte immunoglobulin; n (%) | 1 (10%) | 4 (20%) |
A glucagon-like peptide-1 (GLP-1) agonist (e.g., exenatide 5 mcg subcutaneously within 60 minutes before infusion), was administered and was supposed to be continued (5 mcg BID), for up to 6 months after transplant. Exenatide was not given to the first 4 subjects in Study 1, and 11 of the remaining 26 subjects used exenatide less than the per protocol 6-months post-transplant because of adverse reactions. Because of the variability of exenatide use in the clinical studies, there are insufficient data to support exenatide use in patients receiving LANTIDRA.
Insulin independence, defined as not requiring exogenous insulin to achieve adequate glycemic control, was also determined. Results are summarized in Table 4.
| Total Duration Insulin Independent (years) | N | Mean | Std Dev | Min | Max |
|---|---|---|---|---|---|
| Study 1 | 10 | 5.1 | 4.2 | 0.2 | 12.8 |
| Study 2 | 20 | 3.2 | 3.1 | 0 | 9.9 |
Five subjects had no days of insulin independence. For the 25 subjects who achieved insulin independence, 4 subjects (13.3%) were insulin independent for less than one year, 12 subjects (36.7%) for 1 to 5 years, and 9 subjects (33.3%) for greater than 5 years. Figure 1 shows the entire experience of the individual subjects.
Figure 1: Periods of Insulin Use and Insulin Independence following Initial Infusion, by Patient (Pooled Population)
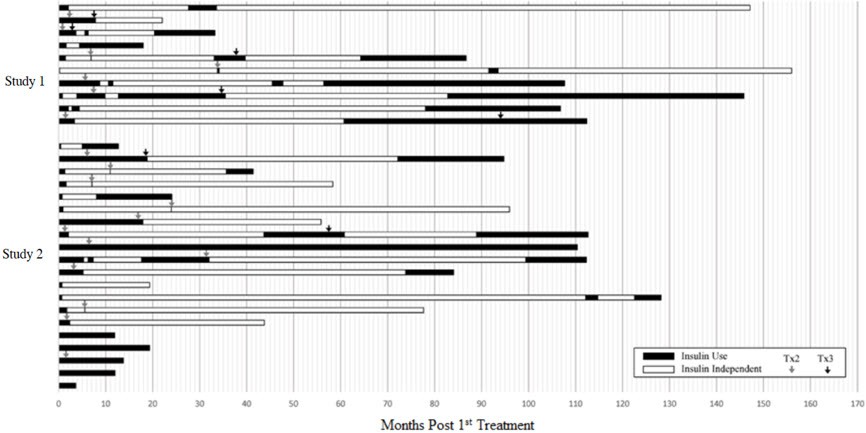
This figure shows the total duration of follow-up for each subject. The period of insulin dependence (use) is denoted in black and the period of insulin independence in white. Time zero (0) is the time of the first infusion. The arrows denote the time of second and third infusions.
16 HOW SUPPLIED/STORAGE AND HANDLING
LANTIDRA (NDC 73539-001-01) is supplied as purified allogeneic islets of Langerhans suspended in buffered transplant medium containing sodium chloride, dextrose, minerals, amino acids, vitamins, and other compounds supplemented with HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid; 10 mM final concentration) and human serum albumin (0.5% final concentration)). [Description (11)].
LANTIDRA is contained in one 1000 mL infusion bag filled with a supplied volume of 400 mL, containing not more than 10 cc of estimated packed islet tissue and not more than 1 × 106 EIN. The 1000 mL infusion bag is aseptically connected to a smaller 750 mL Rinse Bag (NDC 73539-002-01) containing 200 mL of supplied volume of transplant media for use in rinsing the 1000 mL bag containing LANTIDRA and infusion line following infusion to assure complete transfer of islets to the patient. Additional product information, including islet number, is included on the Final Islet Product Certificate of Analysis and the container label.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Prior to prescribing LANTIDRA discuss the following:
Procedural risks
- Portal vein delivery
- liver laceration and hematoma with severe, potentially life-threatening bleeding, which may require prolonged hospitalization and blood transfusions
- liver injury from portal vein thrombosis and possible portal hypertension
- Acute infusion reaction
- symptoms may include fever, chills, fatigue, breathing problems, dizziness, nausea, vomiting, headache, or muscle aches
- a minimum 24-hour stay in the hospital after the procedure will be required for monitoring
Immunosuppression requirements and risks
- Treatment with immunosuppression
- Is required long-term
- If stopped would lead to loss of islet cell function and insulin production
- Can interfere with response to immunizations and that they should avoid live vaccines
- Increased risk of infection
- Infections can be severe and life-threatening
- Infections may require withdraw of immunosuppression
- Development of lymphoma and other malignancies
- Skin malignancies are most common
- Lymphoma and some malignancies may require discontinuation of immunosuppression
- Can interfere with usual response to immunizations
- Patients should receive all appropriate immunizations prior to treatment.
Requirements for ongoing diabetes management and risks
- Not all patients who receive LANTIDRA are able to achieve independence from exogenous insulin (stop insulin injections).
- Not all patients who achieve independence from exogenous insulin can maintain this independence.
- Continued blood glucose monitoring is required after the procedure. Advise patient to follow all instructions regarding glucose monitoring from their endocrinologist and transplant physician.
- Failure to perform continued monitoring can increase the risk of hypoglycemia and hyperglycemia.
- Continued insulin treatment is required after the procedure. Advise patient to follow all instructions regarding insulin dosing from their endocrinologist and transplant physician.
- Failure to continue or restart insulin when required puts patients at risk for severe and potentially life-threatening hyperglycemia, including diabetic ketoacidosis (DKA).
- Patients should seek emergency medical care for severe hypoglycemic episodes and DKA.
Considerations for pregnancy, lactation, and infertility
Pregnancy
- Inform female patients who are of childbearing potential that immunosuppressive drugs required to maintain islet cell survival can cause serious harm, including malformations in the fetus.
- Advise female patients that if they are able to become pregnant, then they should use effective birth control.
- Advise female patients to notify their endocrinologist and transplant physician if they become pregnant.
- Inform male patients receiving LANTIDRA who have female partners who are able to become pregnant that they should use effective birth control before and during treatment.
- If applicable, advise male patients whose partner becomes pregnant, to inform her that she should seek medical advice from her healthcare provider.
Manufactured by:
CellTrans Inc.
2201 W. Campbell Park Drive
Chicago, IL 60612
24-Hour Contact Phone Number: 800-500-1617
© CellTrans
| Patient Information |
| LANTIDRA (donislecel-jujn)
Allogeneic Pancreatic Islet Cellular Suspension for Hepatic Portal Vein Infusion |
| Read this patient information before you start treatment with LANTIDRA. There may be new information. |
| This information does not take the place of talking with your healthcare provider about your medical condition, your treatment options or the potential benefits and risks of treatment with LANTIDRA. |
What is the most important information I should know about LANTIDRA?
LANTIDRA is only for adult patients with Type 1 diabetes who have repeated episodes of severe low blood glucose, those that they need help from someone to treat, and cannot get their HbA1c at the goal set by their endocrinologist and diabetes team, despite intensive diabetes management and education.
LANTIDRA is a cell therapy that is infused (transplanted) into your liver. Talk to your transplant doctor or endocrinologist about your risks from the infusion procedure and the long-term immune suppression medicine that you will need to use after you get the infusion.
Risks from the infusion can include
- damage to the liver with severe bleeding that may require blood transfusions or prolonged hospitalization.
- risk of viruses from the organ donor.
- the infusion may be stopped if the procedure increases pressure in the blood vessels of your liver. If this happens, all of the cells may not be infused.
You will need to take medicines that suppress your immune system regularly for your transplant to survive.
Risks of long-term immune suppression are increased risk of infection, including serious infection, organ failure, and death, and increased risk of certain cancers, including skin and lymph node cancer (lymphoma). Regular follow up appointments are needed.
Call your doctor right away if you have any symptoms of an infection, including:
- fever
- sweats or chills
- cough or flu-like symptoms
- muscle ache
- stiff neck
- warm, red, or painful areas on your skin
- confusion
Follow instructions for regular skin exams and notify your endocrinologist and transplant team if you are told you have skin cancer.
You will need to continue to take insulin and check your blood glucose (sugar) as instructed by your endocrinologist and transplant team.
Insulin independence is not immediate and can take several weeks to occur. Following treatment with LANTIDRA, not every patient becomes insulin independent and some patients who become insulin independent may need to restart insulin.
Monitor your blood glucose levels after getting LANTIDRA. Not all patients are able to stop taking insulin after getting the infusion. Do not stop taking insulin without talking to your doctor. It is very important to follow your doctor's instructions for blood glucose monitoring and keep your follow-up appointments to decrease the chance of serious and life-threatening high glucose or diabetic ketoacidosis.
What is LANTIDRA?
LANTIDRA is an islet cell therapy that is for people with Type 1 Diabetes. Islet cells come from the pancreas of a deceased organ donor.
Pancreatic islet cells include cells, called beta cells, that make insulin. In some people with Type 1 Diabetes, the infused beta cells can make enough insulin to allow the diabetic to control blood glucose without taking insulin.
Who should not take LANTIDRA?
LANTIDRA requires continuing use of medicines that suppress your immune system. Do not get the infusion if you cannot have these medicines because the islet cells will not survive.
Do not get LANTIDRA if you are pregnant or want to become pregnant. Immune suppression medicines can cause serious harm, including death, to you and your developing baby.
If you are male and have a female partner who can become or desires pregnancy, you should ask your transplant team if your immunosuppression drugs can cause abnormal sperm. If your immunosuppression drugs can cause abnormal sperm, advise your female partner to discuss the potential increased risks to her and the developing baby/infant with her healthcare provider.
How will I get LANTIDRA?
LANTIDRA islet cells are infused into your liver through a catheter that is placed into a large blood vessel going into your liver (called the hepatic portal vein). This is done under anesthesia. You will need to stay in the hospital for at least 24 hours.
Before getting LANTIDRA, you will need to start the immune suppression medicine. You will need to continue this medicine after the infusion to keep the islet cells alive.
What should I avoid when I get LANTIDRA?
Because immune suppression can increase your risk of infection, it is important that you:
- follow instructions from your transplant team about avoiding people who have infections, such as colds and flu.
- do not get immunization with live vaccines. Talk to your transplant team before getting any shots to prevent infections.
You can ask your transplant team if there are additional things you should avoid because of your specific immune suppression drugs.
What are the possible or reasonably likely side effects of LANTIDRA?
Injury can occur during the delivery of LANTIDRA into the large blood vessel going to your liver (the hepatic portal vein).
You have a higher risk of infections and cancer because of the immune suppression needed to keep the islet cells alive. In some cases, the immune suppression will be stopped because of these side effects and the islet cells will die and stop making insulin.
You can make antibodies from your islet cell infusion that can make it harder to get a match for transplants, such as a kidney transplant.
You can ask your doctor for information about LANTIDRA that is written for health professionals. Call your doctor about any side effects that concern you.
What should I tell my endocrinologist and transplant physician before receiving LANTIDRA?
For your LANTIDRA (islet cells) to survive, you must strictly follow the instructions for your immune suppression medicines. If you have any questions or problems about taking these medicines, ask your endocrinologist or transplant doctor for help.
If you can become pregnant, you should use effective birth control after getting LANTIDRA. Talk with your doctor about the birth control regimens that may be right for you.
Some immunosuppressive drugs may cause formation of abnormal sperm. Ask your doctor if your immunosuppression drugs can cause abnormal sperm. If so, and you have a female partner who can become pregnant, you should discuss this with your partner and use effective birth control before starting treatment with immunosuppression drugs.
What are the ingredients of LANTIDRA?
In addition to the cells in LANTIDRA the delivery fluid contains:
CaC12, anhydrous, biotin, MgSO4, anhydrous, folic acid, sodium acetate, anhydrous, riboflavin, NaH2PO4H2O, Cocarboxylase, dextrose, Li3 Coenzyme A 2 H2O, KCl, Cozymase, NaCl, Na2 Flavin adenine dinucleotide, Na Gluconate H2O, Na Triphosphopyridine Nucleotide, L-alanine, Na3 Uridine 5'-Triphosphoric Acid H2O, L-arginine HCl, ascorbic acid, L-aspartic acid, D-Ca-Pantothenate, L-cysteine HCl H2O, choline chloride, L-cystine 2 HCl, i-inositol, L-glutamic acid, nicotinic acid, glycine, nicotinamide, L-histidine HCl H2O, para-aminobenzoic acid, Hydroxy-L-proline, pyridoxine HCl, L-isoleucine, thiamine HCl, L-leucine, glutathione (reduced), L-lysine HCl, thymidine, L-methionine, 2D-adenosine, L-phenylalanine, 2D-cytidine HCl, L-proline, 2D-guanosine, L-serine, 5-methyl-2'- deoxycytidine, L-threonine, cholesterol, L-tryptophan, Tween 80, L-valine, L-alanyl-L-glutamine, L-tyrosine 2 Na 2 H2O
Name and Place of manufacturer:
CellTrans Inc.
1740 W. Taylor St., STE C200
Chicago, IL, 60612
24-Hour Contact Phone Number: 800-500-1617
PRINCIPAL DISPLAY PANEL - LANTIDRA Bag Label
donislecel – jujn
LANTIDRA
Allogeneic Pancreatic Islets, Cellular Suspension for Hepatic Portal Infusion.
Dose: One Sterile Bag of LANTIDRA for Primary Infusion followed by
One Sterile bag (Rinse) for secondary infusion
Rx Only
Lot Number: ______
Total EIN: ________
Manufactured Date:
YYYY–MMM–DD
Time
__:__
Product needs to be administered within 6 hours of product release time
For Use by Intended Recipient Only
Recipient Name: ________________
Recipient ID: ___________________
DOB: YYYY–MMM–DD
Recipient ABO/Rh: __/__
Do Not Use a Leukodepleting Filter
Do Not Irradiate
Storage at Room Temperaature (15°–25°C)
Transplant within expiration date and time,
LANTIDRA bag primary infusion
Target dose is 5,000 EIN/kg for initial transplant and
4,500 EIN/kg for subsequent transplants. LANTIDRA is a
cellular suspension of allogeneic pancreatic islets (Islets
of Langerhans) in buffered transplant media (See package
insert for complete list of transplant medium ingredients).
Donor Identifier
Donor ID: __________
DOB: YYYY–MMM–DD
Donor ABO/Rh: __/__
Dispose of Used Material that comes in
contact with LANTIDRA as biohazard
waste in accordance with local requirements
NDC 73539–001–01
Manufactured by: CellTrans Inc.
1740 W. Taylor St., STE C200,
Chicago, IL, 60612 (P: + 1 312 413 3507)

PRINCIPAL DISPLAY PANEL - RINSE Bag Label
Rinse Bag for Infusion following hepatic
portal infusion of LANTIDRA (Allogeneic
Pancreatic Islets for hepatic portal infusion)
Dose: One Sterile Bag of Rinse for Secondary
Infusion after primary infusion of LANTIDRA.
Rx Only
Lot Number: ______
Total EIN: ________
Manufactured Date:
YYYY–MMM–DD
Time
__:__
Product needs to be administered within 6 hours of product release time
Rinse Bag For Intended Recipient Only
(ONLY FOR USE WITH LANTIDRA)
Recipient Name: ________________
Recipient ID: ___________________
DOB: YYYY–MMM–DD
Recipient ABO/Rh: __/__
Do Not Use a Leukodepleting Filter
Do Not Irradiate
Storage at Room Temperaature (15°–25°C)
Transplant within expiration date and time
Rinse bag infused after the primary LANTIDRA
bag infusion and rinse goes through the LANTIDRA
CryoMACS bag
Target total volume of Rinse: 200 ml per bag. Transplant
media used for rinsing LANTIDRA CryoMACS bag to ensure
all islets are transfused. Rinse/Transplant media ingredient
list (See package insert for complete list of transplant
medium ingredients).
Dispose of Used Material that comes in
contact with LANTIDRA as biohazard
waste in accordance with local requirements
NDC 73539–002–01
Manufactured by: CellTrans Inc.
1740 W. Taylor St., STE C200,
Chicago, IL, 60612 (P: + 1 312 413 3507)
PRINCIPAL DISPLAY PANEL - Secondary Container Label
CONTENTS WITHIN THE OVERWRAP:
One sterile bag for Allogeneic Islet Cell
Suspension for hepatic portal infusion (LANTIDRA)
attached to one sterile bag for Rinse infusion.

PRINCIPAL DISPLAY PANEL - Carrier Label
Manufactured by: CellTrans Inc.
1740 W. Taylor St., STE C200,
Chicago, IL, 60612
Phone: + 1 312 413 3507
Contents of Carrier System:
–LANTIDRA CryoMACS bag attached to Rinse CryoMACS bag within a primary and a secondary overwrap
–Package Insert
–Drape covers
–Digital thermometer
Accompanying Documentation:
–Donor Eligibility Determination Summary Records
–Final Islet Product Certificate of Analysis
–Final Product, Chain of Custody Form
Affixed Labels:
–LANTIDRA CryoMACS bag labels affixed to the CryoMACS bag containing the Allogeneic Islet Cell Suspension, to
the primary overwrap and to the secondary overwrap
–Rinse CryoMACS bag labels affixed to the CryoMACS bag containing 200 mL Transplant Media, to the primary
overwrap and to the secondary overwrap
Details of Transport:
The transport carrier containing LANTIDRA CryoMACS bag and Rinse CryoMACS bag is transported by walking from
CellTrans to UIHealth, Department of Radiology, located on the second floor (accessed by elevator) of the same
building where CellTrans is located. The entire transportation time is less than 5 minutes.
Address/Location of Destination: 1740 W. Taylor Street, UIHealth, Department of Radiology, Angiography,
Room 2471–2479.
