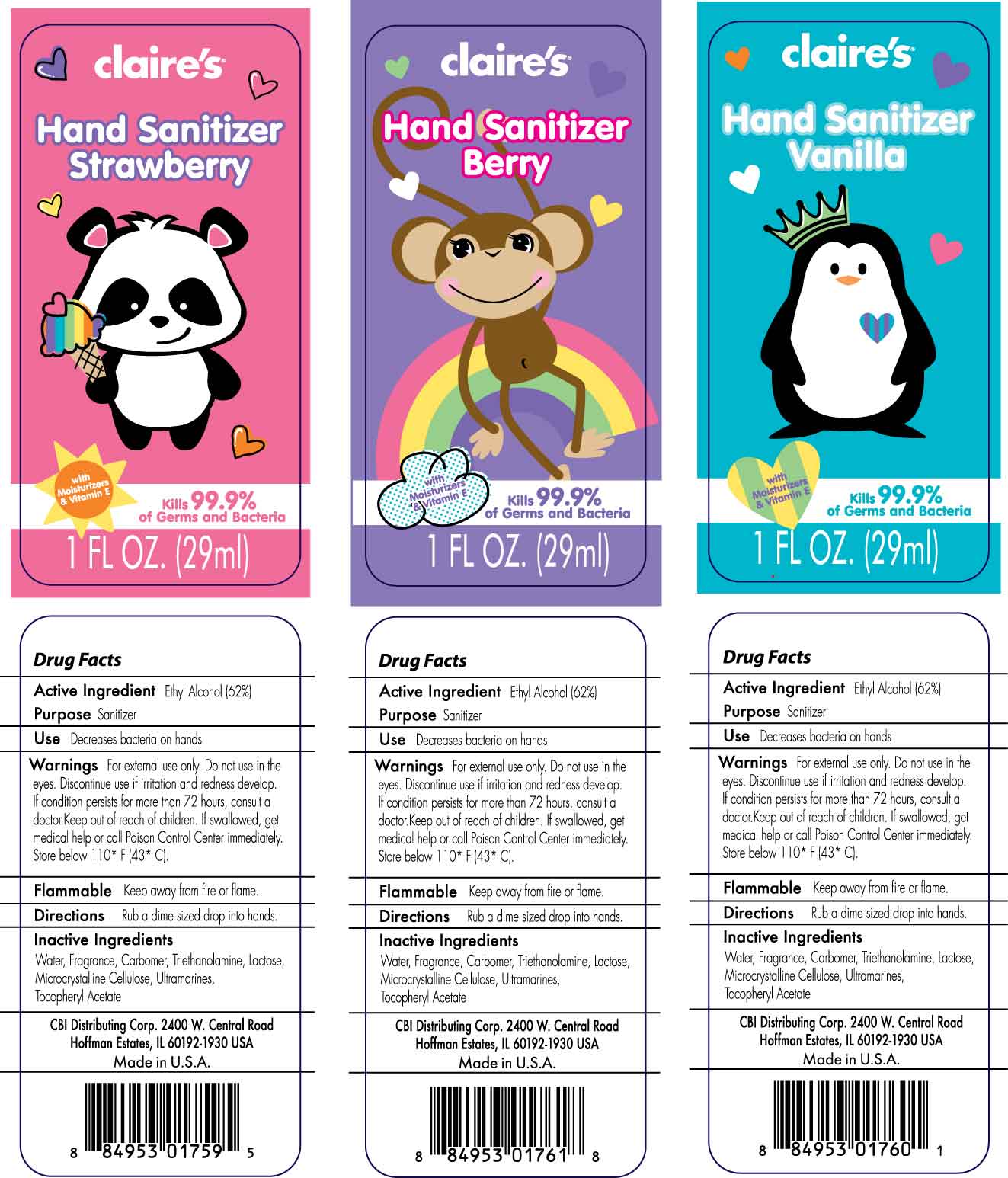
CLAIRES - hand sanitizer gel
Yusef Manufacturing Laboratories, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Use Decreases bacteria on hands
Warnings For external use only. Do not use in the eyes. Discontinue use if irritation and redness develop. If condition persists more than 72 hours, consult a doctor. Keep out of reach of children. If swallowed, get medical help or call Poison Control Center immediately. Store below 110 F (43 C).
Flammable Keep away from fire or flame.
Directions Rub a dime size drop into hands.
Inactive Ingredients Water, Fragrance, Carbomer, Triethanolamine, Lactose, Microcrystalline Cellulose, Ultramarines, Tocopheryl Acetate
CBI Distributing Corp. 2400 W. Central Road
Hoffman Estates, IL 60192-1930 U.S.A.
 Enter section text here
Enter section text here
 Enter section text here
Enter section text here