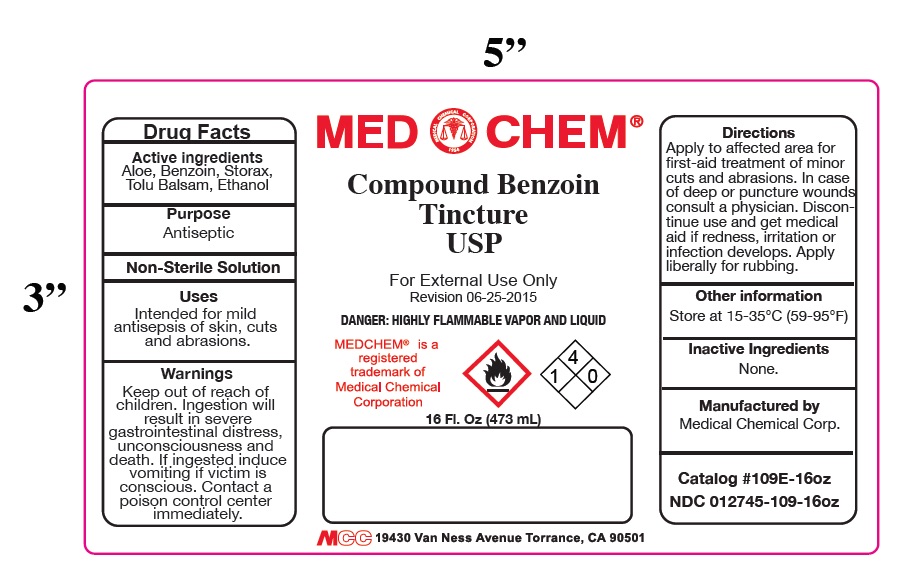
Warnings: Keep out of reach of children. Ingestion will result in severe gastrointestinal distress, unconsciousness and death. If ingested induce vomiting if the victim is conscious. Contact a poison control center immediately.
Directions: Apply to affected area for first-aid treatment of minor cuts and abrasions. Discontinue use and get medical aid if redness, irritation or infection develops. In case of deep or puncture wounds consult a physician.
Warnings: Keep out of reach of children. Ingestion will result in a severe gastrointestinal distress, unconsciousness and death. If ingested induce vomiting if victim is conscious. Contact a poison control center immediately.