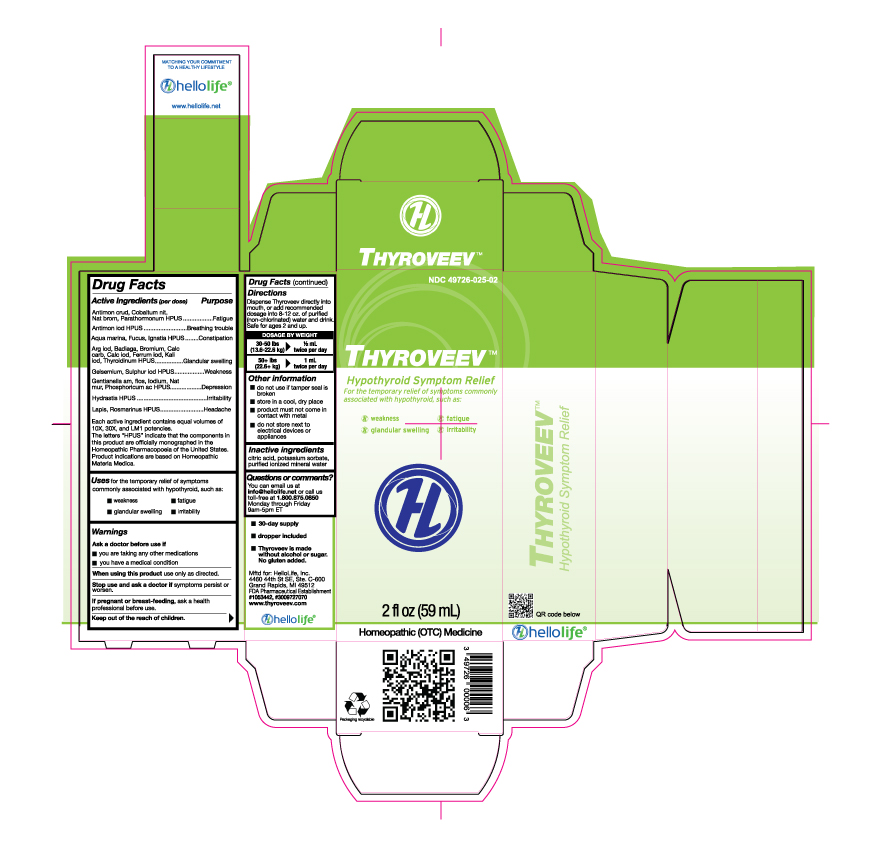
Active Ingredients (per dose)
Antimon crud, Cobaltum nit, Nat brom HPUS, Parathormonum HPUS
Antimon iod HPUS
Aqua marina, Fucus, Ignatia HPUS
Arg iod, Badiaga, Bromium, Calc carb, Calc iod, Ferrum iod, Kali iod, Thyroidinum HPUS
Gelsemium, Sulphur iod HPUS
Gentianella am, flos, Iodium, Nat mur, Phosphoricum ac HPUS
Hydrastis HPUS
Lapis, Rosmarinus HPUS
Each active ingredient contains equal volumes of 10X, 30X, and LM1 potencies. The letter "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. Product indications are based on Homeopathic Materia Medica.
Purpose
Antimon crud, Cobaltum nit, Nat brom, Parathormonum HPUS...........................................................................Fatigue
Antimon iod HPUS..........................................................................................................................................Breathing trouble
Aqua marina, Fucus, Ignatia HPUS...................................................................................................................Constipation
Arg iod, Badiaga, Bromium, Calc carb, Calc iod, Ferrum iod, Kali iod, Thyroidinum HPUS.....................................Glandular swelling
Gelsemium, Sulphur iod HPUS.........................................................................................................................Weakness
Gentianella am, flos, Iodium, Nat mur, Phosphoricum ac HPUS...........................................................................Depression
Hydrastis HPUS...............................................................................................................................................Irritability
Lapis, Rosmarinus HPUS..................................................................................................................................Headache
Uses
For the temporary relief of symptoms commonly associated with hypothyroid, such as:
- weakness
- fatigue
- glandular swelling
- irritability
Warnings
Ask a doctor before use if
|
Directions
| Dispense Thyroveev directly into mounth, or add recommended dosage into 8-12 oz purified (non-chlorinated) water and
drink. Safe for ages 2 and up.
DOSAGE BY WEIGHT 30-50 lbs (13.6-22.6 kg) 1/2 mL twice per day 50+ lbs (22.6+ kg) 1 mL twice per day |
Other information
- do not use if tamper seal is broken
- store in a cool, dry place
- product must not come in contact with metal
- do not store next to electrical devices or appliances
Questions or comments?
| You can email us at info@hellolife.net or call us toll-free at 1.800.875.0850 Monday through Friday 9am-5pm ET |