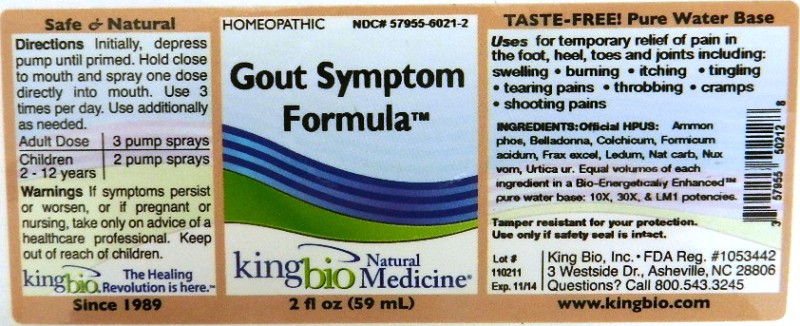
Active Ingredients
Official HPUS
Ammonium Phosphoricum, Belladonna, Colchicum Autumnale, Formicum Acidum, Fraxinus Excelsior, Ledum Palustre, Natrum Carbonicum, Nux Vomica, and Urtica Urens.
Reference image gout.jpg
Inactive Ingredient
Equal volumes of each ingredient in a Bio-Energetically Enhanced pure water base. 10X, 30X, and LM1 potencies.
Reference image gout.jpg
Dosage and Administration
Directions Initially, depress pump until primed. Hold close to mouth and spray one dose directly into mouth. Use 3 times per day. Use additionally as needed.
Adult Dose: 3 pump sprays
Children: (2-12 years) 2 pump sprays
Reference image gout.jpg
Purpose
For Temporary relief of pain in the foot, heel, toes and joints including: swelling, burning, itching, tingling, tearing pains, throbbing, cramps, shooting pains.
Reference image gout.jpg
Warnings
If symptoms persist or worsen, or if pregnant or nursing, take only on advice of a healthcare professional. Keep out of reach of children.
Other: Tamper resistant for your protection. Use only if safety seal is intact.
Reference image gout.jpg