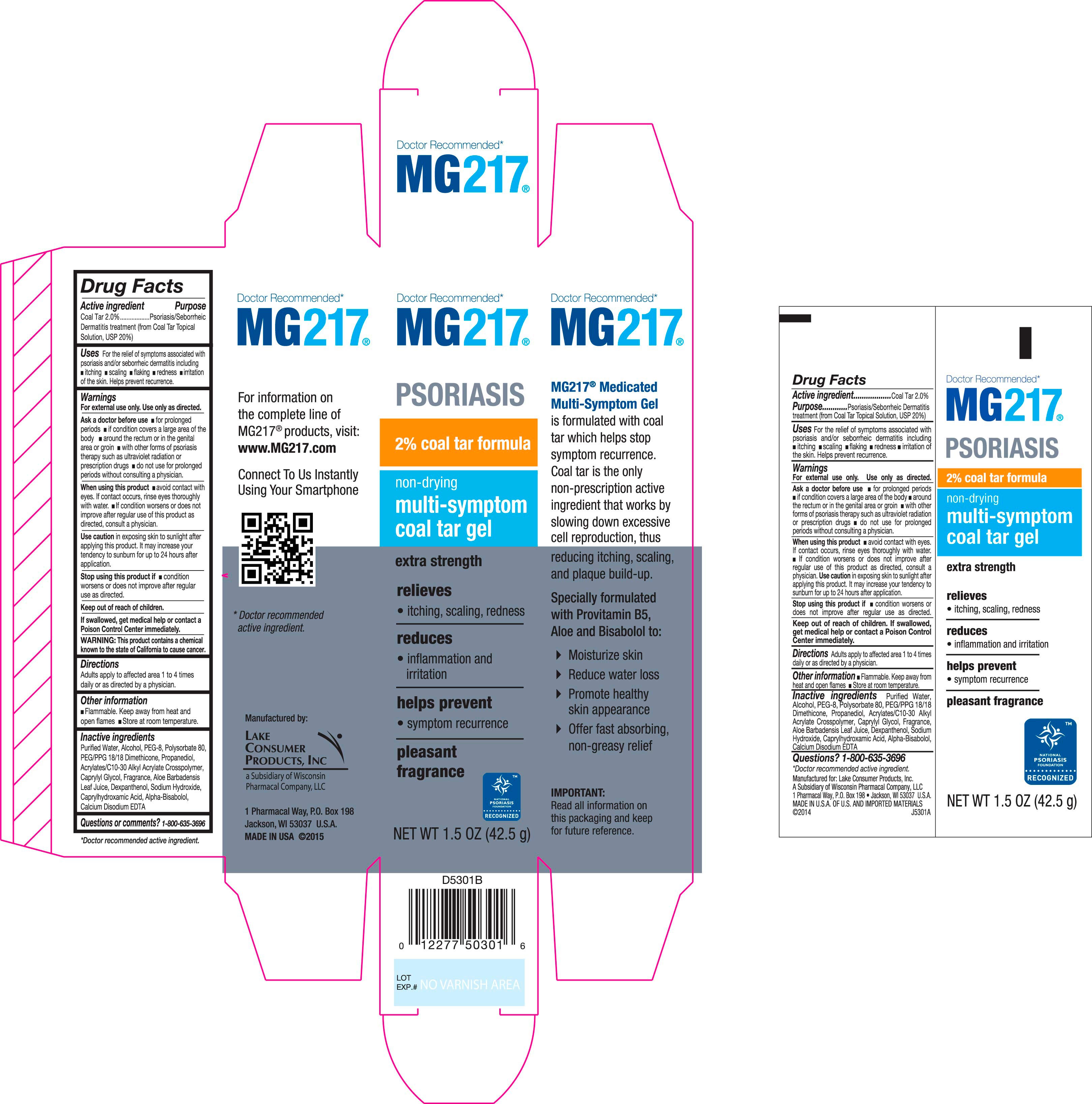
Purpose
Psoriasis, Seborrheic Dermatitis, Dandruff Treatment (from Coal Tar Topical Solution, USP 20%)
Uses
For the temporary relief of these symptoms associated with psoriasis and/or seborrheic dermatitis including,
- itching
- scaling
- flaking
- redness
- irritation of the scalp
Helps prevent recurrence.
Warnings
For external use only. Use only as directed.
Ask doctor before use
- for prolonged periods
- if condition covers a large area of the body.
- around the rectum or in the genital area or groin
- with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs
- do not use for prolonged periods without consulting a physician
When using this product
- avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water.
- If condition worsens or does not improve after regular use of this product as directed, consult a physician.
- Use caution in exposing skin to sunlight. It may increase your tendency to sunburn for up to 24 hours after application
Inactive ingredients
Purified Water, Alcohol, PEG-8, Polysorbate 80, PEG/PPG 18/18 Dimethicone, Propanediol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylyl Glycol, Fragrance, Aloe Barbadensis Leaf Juice, Dexpanthenol, Sodium Hydroxide, Caprylhdroxamic Acid, Alpha-Bisabolol, Calcium Disodium EDTA
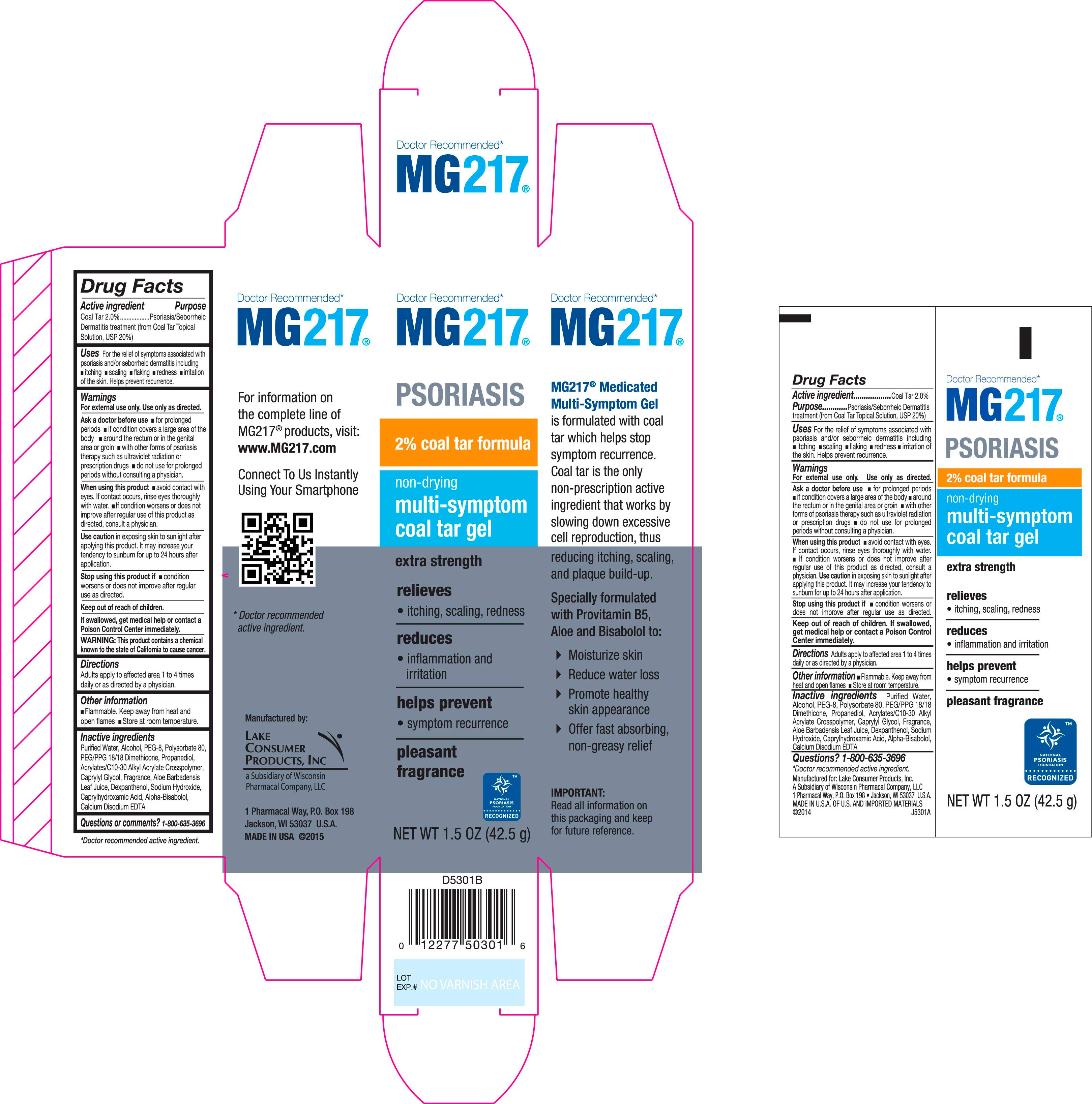 Carton and Label
Carton and Label