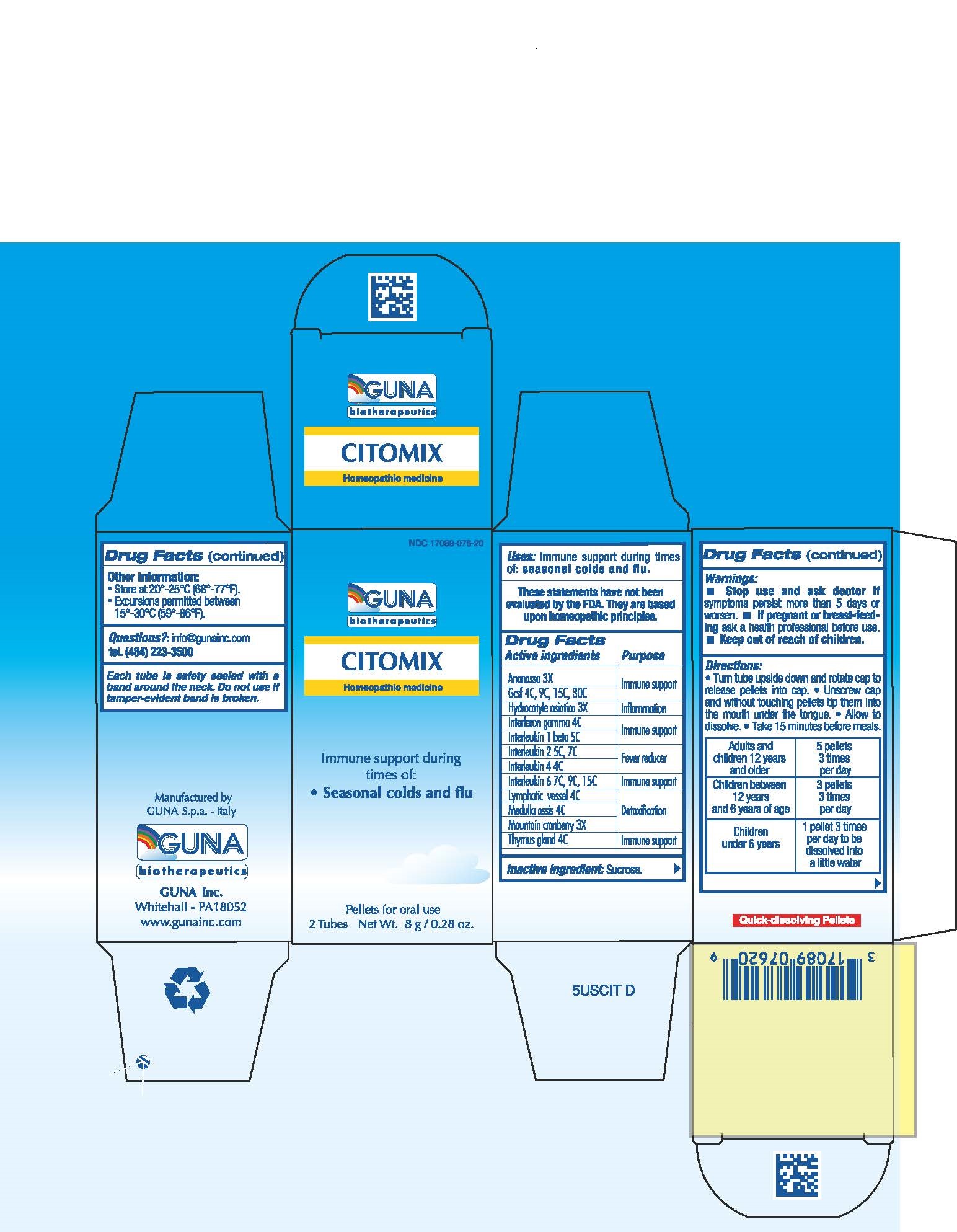
ACTIVE INGREDIENTS/PURPOSE
ANANASSA 3X IMMUNE SUPPORT
GCSF 4C, 9C, 15C, 30C IMMUNE SUPPORT
HYDROCOTYLE ASIATICA 3X INFLAMMATION
INTERFERON GAMMA 4C IMMUNE SUPPORT
INTERLEUKIN 1 BETA 5C IMMUNE SUPPORT
INTERLEUKIN 2 5C, 7C FEVER REDUCER
INTERLEUKIN 4 4C FEVER REDUCER
INTERLEUKIN 6 7C, 9C, 15C IMMUNE SUPPORT
LYMPHATIC VESSEL 4C DETOXIFICATION
MEDULLA OSSIS 4C DETOXIFICATION
MOUNTAIN CRANBERRY 3X DETOXIFICATION
THYMUS GLAND 4C IMMUNE SUPPORT
DIRECTIONS
- Turn tube upside down and rotate cap to release pellets into cap.
- Unscrew cap and without touching pellets tip them into the mouth under the tongue.
- Allow to dissolve
- Take 15 minutes before meals.
- Take 15 minutes before meals
- Adults and children 12 years and older 5 pellets 3 times per day
- Children between 12 years and 6 years of age 3 pellets 3 times per day
- Children under 6 years 1 pellet 3 times per day to be disoolved into a little water