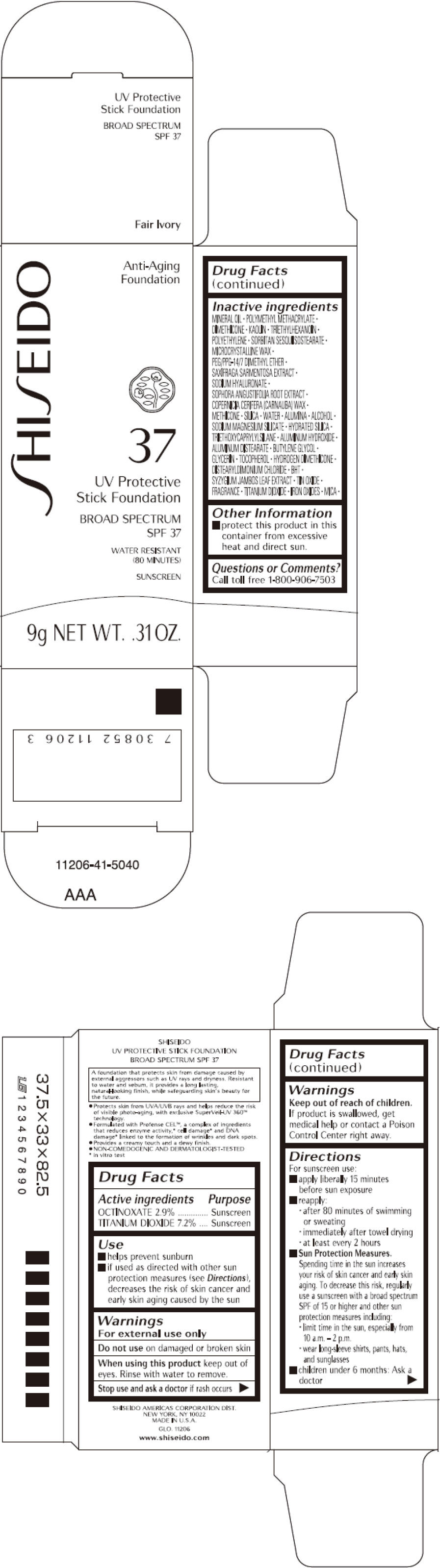
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
MINERAL OIL, POLYMETHYL METHACRYLATE, DIMETHICONE, KAOLIN, TRIETHYLHEXANOIN, POLYETHYLENE, SORBITAN SESQUIISOSTEARATE, MICROCRYSTALLINE WAX, PEG/PPG-14/7 DIMETHYL ETHER, SAXIFRAGA SARMENTOSA EXTRACT, SODIUM HYALURONATE, SOPHORA ANGUSTIFOLIA ROOT EXTRACT, COPERNICIA CERIFERA (CARNAUBA) WAX, METHICONE, SILICA, WATER, ALUMINA, ALCOHOL, SODIUM MAGNESIUM SILICATE, HYDRATED SILICA, TRIETHOXYCAPRYLYLSILANE, ALUMINUM HYDROXIDE, ALUMINUM DISTEARATE, BUTYLENE GLYCOL, GLYCERIN, TOCOPHEROL, HYDROGEN DIMETHICONE, DISTEARYLDIMONIUM CHLORIDE, BHT, SYZYGIUM JAMBOS LEAF EXTRACT, TIN OXIDE, FRAGRANCE, TITANIUM DIOXIDE, IRON OXIDES, MICA,
PRINCIPAL DISPLAY PANEL - 9 g Cartridge Carton - Fair Ivory
SHI SEIDO
Anti-Aging
Foundation
37
UV Protective
Stick Foundation
BROAD SPECTRUM
SPF 37
WATER RESISTANT
(80 MINUTES)
SUNSCREEN
9g NET WT. .31 OZ.
PRINCIPAL DISPLAY PANEL - 9 g Cartridge Carton - Fair Ochre
SHI SEIDO
Anti-Aging
Foundation
37
UV Protective
Stick Foundation
BROAD SPECTRUM
SPF 37
WATER RESISTANT
(80 MINUTES)
SUNSCREEN
9g NET WT. .31 OZ.


