FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
UCERIS rectal foam is indicated for the induction of remission in patients with active mild to moderate distal ulcerative colitis extending up to 40 cm from the anal verge.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage regimen is 1 metered dose administered rectally twice daily for 2 weeks followed by 1 metered dose administered rectally once daily for 4 weeks.
2.2 Administration Instructions
Advise patients:
- •
- UCERIS rectal foam is only to be applied rectally. It is not for oral use.
- •
- Before using UCERIS rectal foam, use the bathroom to empty your bowels.
- •
- Each applicator is coated with a lubricant. If additional lubrication is needed, petrolatum or petroleum jelly can also be used.
- •
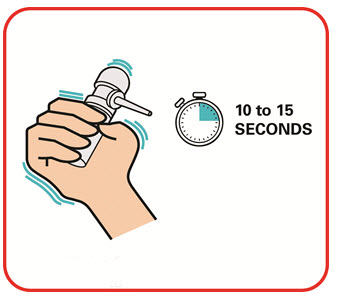
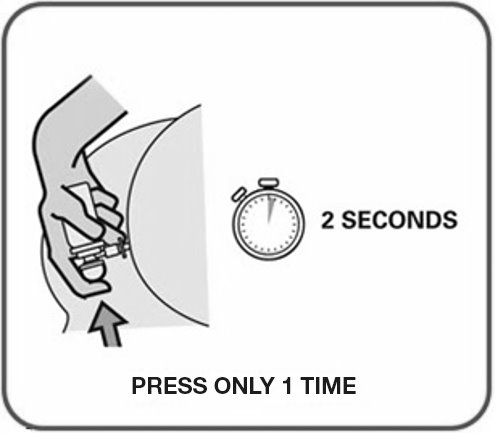
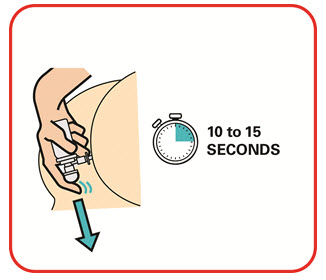
- Warm the canister in the hands while shaking it vigorously for 10 to 15 seconds prior to use.
- •
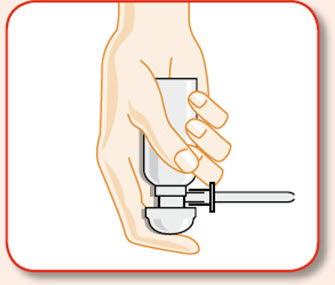
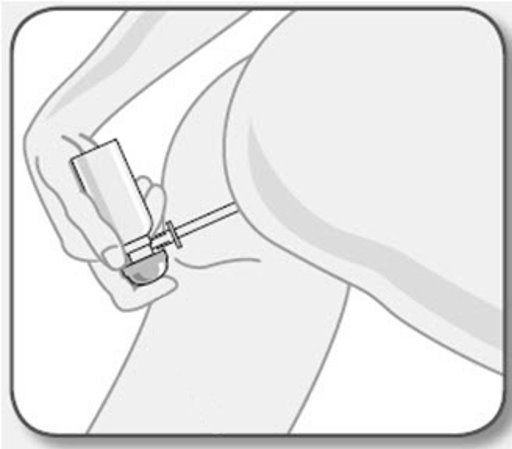
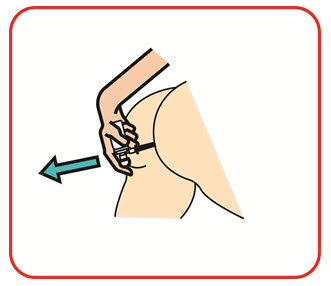
- UCERIS rectal foam can be used in a standing, lying or sitting position (e.g., while using the toilet).
- •
- Apply UCERIS rectal foam in the morning and the evening for the first 2 weeks of treatment; then once daily in the evening for the next 4 weeks. When applied in the evening, use immediately prior to bedtime. Try not to empty your bowels again until the next morning.
- •
- Avoid concomitant use of CYP3A4 inhibitors (e.g., ketoconazole, grapefruit juice) during treatment with UCERIS rectal foam.
3 DOSAGE FORMS AND STRENGTHS
UCERIS rectal foam is formulated as an emulsion which is filled into an aluminum canister with an aerosol propellant. It is available in 1 strength: 2 mg budesonide per metered dose.
4 CONTRAINDICATIONS
UCERIS rectal foam is contraindicated in patients with a history of a known hypersensitivity to budesonide or any of the ingredients of UCERIS rectal foam. Reactions have included anaphylaxis [see Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypercorticism and Adrenal Axis Suppression
When glucocorticosteroids are used chronically, systemic effects such as hypercorticism and adrenal suppression may occur. Glucocorticosteroids can reduce the response of the hypothalamus-pituitary-adrenal (HPA) axis to stress. In situations where patients are subject to surgery or other stress situations, supplementation with a systemic glucocorticosteroid is recommended. Since UCERIS rectal foam contains a glucocorticosteroid, general warnings concerning glucocorticoids should be followed [see Clinical Pharmacology (12.2)].
Reduced liver function affects the elimination of glucocorticosteroids, and increased systemic availability of oral budesonide has been demonstrated in patients with liver cirrhosis [see Use in Specific Populations (8.6)].
5.2 Impaired Adrenal Suppression in Patients Transferred from Other Glucocorticoids
Monitor patients who are transferred from glucocorticosteroid treatment with higher systemic effects to glucocorticosteroids with lower systemic effects, such as UCERIS rectal foam, since symptoms attributed to withdrawal of steroid therapy, including those of acute adrenal suppression or benign intracranial hypertension, may develop. Adrenocortical function monitoring may be required in these patients and the dose of glucocorticosteroid treatment with high systemic effects should be reduced cautiously.
Replacement of systemic glucocorticosteroids with UCERIS rectal foam may unmask allergies (e.g., rhinitis and eczema), which were previously controlled by the systemic drug.
5.3 Increased Risk of Infection
Patients who are on drugs that suppress the immune system are more susceptible to infection than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible patients or patients on immunosuppressant doses of glucocorticosteroids. In patients who have not had these diseases, particular care should be taken to avoid exposure.
How the dose, route and duration of glucocorticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior glucocorticosteroid treatment to the risk is also not known. If exposed, therapy with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG), as appropriate, may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (see prescribing information for VZIG and IG). If chicken pox develops, treatment with antiviral agents may be considered.
Glucocorticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection, untreated fungal, bacterial, systemic viral or parasitic infections, or ocular herpes simplex.
5.4 Other Glucocorticosteroid Effects
Monitor patients with hypertension, diabetes mellitus, osteoporosis, peptic ulcer, glaucoma or cataracts, or with a family history of diabetes or glaucoma, or with any other condition where glucocorticosteroids may have unwanted effects.
5.5 Flammable Contents
The contents of UCERIS rectal foam include n-butane, isobutane and propane as propellants which are flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following administration. Patients should temporarily discontinue use of UCERIS rectal foam before initiation of bowel preparation for colonoscopy and consult their healthcare provider before resuming therapy.
6 ADVERSE REACTIONS
Serious and important adverse reactions include:
- •
- Hypercorticism and adrenal axis suppression [see Warnings and Precautions (5.1)]
- •
- Symptoms of steroid withdrawal in those patients transferring from systemic glucocorticosteroid therapy [see Warnings and Precautions (5.2)]
- •
- Increased susceptibility to infection [see Warnings and Precautions (5.3)]
- •
- Other glucocorticosteroid effects [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to UCERIS rectal foam in 332 patients with active mild to moderate distal ulcerative colitis extending up to 40 cm from the anal verge. The median duration of exposure was 42 days. This included 14 patients exposed for at least 6 months.
UCERIS rectal foam was studied primarily in 2 placebo-controlled, 6-week trials in patients with active disease (Study 1 and Study 2). In these trials, 268 patients received UCERIS rectal foam 2 mg twice a day for 2 weeks followed by 2 mg once a day for 4 weeks [see Clinical Studies (14)].
The most common adverse reactions (≥ 2% of the UCERIS rectal foam or Placebo group and at higher frequency in the UCERIS rectal foam group) were decreased blood cortisol, adrenal insufficiency, and nausea (Table 1). Decreased blood cortisol was defined as a morning cortisol level of <5 mcg/dL. Adrenal insufficiency was defined as a cortisol level of <18 mcg/dL at 30 minutes post-challenge with adrenocorticotropic hormone (ACTH).
A total of 10% of UCERIS rectal foam-treated patients discontinued treatment due to an adverse reaction compared with 4% of placebo-treated patients.
|
Adverse Reaction |
UCERIS Rectal Foam
|
Placebo
|
|
Decreased blood cortisol |
46 (17) |
6 (2) |
|
Adrenal insufficiency† |
10 (4) |
2 (1) |
|
Nausea |
6 (2) |
2 (1) |
Of the 46 UCERIS rectal foam treated patients with decreased blood cortisol (defined as a morning cortisol level of <5 mcg/dL) reported as an adverse event, none had adrenal insufficiency (defined as a cortisol level of <18 mcg/dL at 30 minutes post-challenge with ACTH) (see Table 2). All cases of adrenal insufficiency resolved.
Table 2 summarizes the percentages of patients reporting glucocorticoid related effects in the 2 placebo-controlled trials (Studies 1 and 2).
- Table 2: Summary of Glucocorticoid Related Effects in Two Placebo-Controlled Trials
- (Studies 1 and 2)
|
Adverse Reaction |
UCERIS Rectal Foam
|
Placebo
|
|
Overall |
60 (22) |
10 (4) |
|
Blood cortisol decreased |
46 (17)* |
6 (2) |
|
Adrenal insufficiency |
10 (4) |
2 (1) |
|
Insomnia |
1 (0.4) |
1 (0.4) |
|
Sleep disorder |
1 (0.4) |
0 |
|
Acne |
1 (0.4) |
0 |
|
Depression |
1 (0.4) |
1 (0.4) |
|
Hyperglycemia |
1 (0.4) |
0 |
No clinically significant differences were observed with respect to the overall percentages of patients with any glucocorticoid related effects between UCERIS rectal foam and placebo after 6 weeks of therapy.
For additional details on morning cortisol levels and the response to the ACTH stimulation test, see Clinical Pharmacology (12.2).
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials for UCERIS rectal foam, the following adverse reactions have been identified during post-approval use of other oral and rectal formulations of budesonide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: hypertension
Gastrointestinal disorders: pancreatitis
General disorders and administration site conditions: pyrexia, peripheral edema
Immune system disorders: anaphylactic reactions
Nervous system disorders: dizziness, benign intracranial hypertension
Psychiatric disorders: mood swings
Skin and subcutaneous tissue disorders: pruritus, maculopapular rash, allergic dermatitis
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
The active ingredient of UCERIS rectal foam, budesonide, is metabolized by CYP3A4. Inhibitors of CYP3A4 activity (such as ketoconazole, itraconazole, ritonavir, indinavir, saquinavir, erythromycin, cyclosporine and grapefruit juice) can increase systemic budesonide concentrations. Avoid concomitant use of CYP3A4 inhibitors with UCERIS rectal foam [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published studies report on the use of budesonide in pregnant women; however, the data are insufficient to inform a drug-associated risk for major birth defects and miscarriage. There are clinical considerations (see Clinical Considerations). In animal reproduction studies with pregnant rats and rabbits, subcutaneous administration of budesonide during organogenesis at doses 1.2 times and 0.12 times, respectively, the human intrarectal dose of 4 mg/day, resulted in increased fetal loss, decreased pup weights, and skeletal abnormalities. Maternal toxicity was observed in both rats and rabbits at these dose levels (see Data). Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage of the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is of 2% to 4%, and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with ulcerative colitis. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
Fetal/Neonatal Adverse Reactions
Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Infants should be carefully observed for signs of hypoadrenalism, such as poor feeding, irritability, weakness, and vomiting, and managed accordingly [see Warnings and Precautions (5.1)].
Data
Animal Data
Budesonide was teratogenic and embryolethal in rabbits and rats.
In an embryo-fetal development study in pregnant rats dosed subcutaneously with budesonide during the period of organogenesis from gestation days 6-15 there were effects on fetal development and survival at subcutaneous doses up to approximately 500 mcg/kg in rats (approximately 1.2 times the recommended human intrarectal dose on a body surface area basis). In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6-18, there was an increase in maternal abortion, and effects on fetal development and reduction in litter weights at subcutaneous doses up to approximately 25 mcg/kg in rabbits (approximately 0.12 times the recommended human intrarectal dose of 4 mg/day on a body surface area basis). Maternal toxicity, including reduction in body weight gain, was observed at subcutaneous doses of 5 mcg/kg in rabbits (approximately 0.02 times the recommended human intrarectal dose on a body surface area basis) and 500 mcg/kg in rats (approximately 1.2 times the recommended human intrarectal dose of 4 mg/day on a body surface area basis).
In a pre-and post-natal development study, rats dosed subcutaneously with budesonide during the period of Day 15 post coitum to Day 21 postpartum, budesonide had no effects on delivery but did have an effect on growth and development of offspring. In addition, offspring survival was reduced, and surviving offspring had decreased mean body weights at birth and during lactation at exposures 0.05 times the MRHD (on a mg/m2 basis at maternal subcutaneous doses of 20 mcg/kg/day and higher). These findings occurred in the presence of maternal toxicity.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted with UCERIS rectal foam or other rectally administered budesonide products and no information is available on the effects of budesonide on the breastfed infant or the effects of the drug on milk production. One published study reports that budesonide is present in human milk following maternal inhalation of budesonide (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for UCERIS rectal foam and any potential adverse effects on the breastfed child from UCERIS rectal foam or from the underlying maternal condition. Data
One published study reports that budesonide is present in human milk following maternal inhalation of budesonide and the milk/plasma ratio ranged between 0.4 and 0.5. Budesonide plasma concentrations were not detected, and no adverse events were noted in the breastfed infants following maternal use of inhaled budesonide. The systemic exposure (AUC0-12) following administration of 400 mcg twice a day of inhaled budesonide ranged from 1.27 to 2.26 ng*hr/mL. The estimated budesonide AUC0-12 following rectal administration of 2 mg twice a day UCERIS was 4.31 ng*hr/mL [see Clinical Pharmacology (12.3)]. Budesonide exposure to the nursing child may be higher with maternal rectal administration of UCERIS than with maternal inhaled administration of budesonide.
8.4 Pediatric Use
The safety and effectiveness of UCERIS rectal foam has not been established in pediatric patients.
Children who are treated with corticosteroids by any route may experience a decrease in their growth velocity. This negative impact of corticosteroids on growth has been in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression. The long-term effects of this reduction in growth velocity associated with corticosteroid treatment, including the impact on final adult height, are unknown. Growth velocity may therefore be a more sensitive indicator of systemic corticosteroid exposure in children than some commonly used tests of HPA axis function. The linear growth of children treated with corticosteroids by any route should be monitored (e.g., via stadiometry), and the potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of other treatment alternatives. In order to minimize the potential growth effects of corticosteroids, children should be titrated to the lowest effective dose.
8.5 Geriatric Use
Clinical studies with UCERIS rectal foam did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
No dosage adjustment is needed for patients with mild (Child-Pugh Class A) hepatic impairment. Patients with moderate to severe hepatic impairment (Child-Pugh Class B or C) should be monitored for increased signs and/or symptoms of hypercorticism. Discontinuing the use of UCERIS rectal foam should be considered in these patients if signs of hypercorticism are observed [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Acute overdosage with UCERIS rectal foam is unlikely. However, UCERIS rectal foam is absorbed systemically and chronic overdosage may result in signs/symptoms of hypercorticism [see Warnings and Precautions (5.1)].
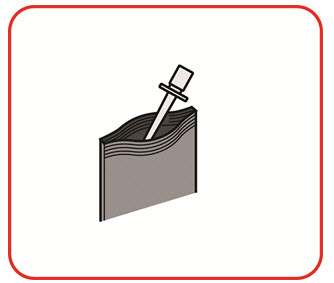
11 DESCRIPTION
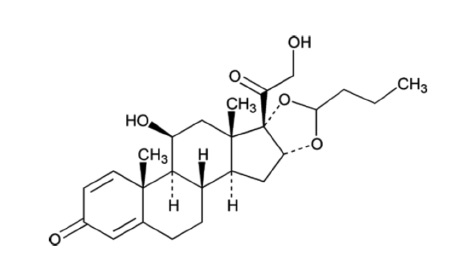
UCERIS rectal foam contains budesonide, a non-halogenated synthetic glucocorticoid, as the active ingredient. It is a mixture of the 2 epimers (22R and 22S) differing in the position of an acetal chain. Both epimers are active glucocorticoids applied in a mixture of approximately 1:1.
Budesonide is designated chemically as (RS)-11β, 16α, 17,21 tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula is:
UCERIS rectal foam contains 2 mg budesonide per metered dose.
Inactive ingredients: cetyl alcohol, citric acid monohydrate, edetate disodium, emulsifying wax, polyoxyl (10) stearyl ether, propylene glycol, and purified water.
Propellant: n-butane, isobutane, and propane.
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Treatment with glucocorticosteroids, including UCERIS rectal foam, is associated with a suppression of endogenous cortisol concentrations and an impairment of the hypothalamic-pituitary-adrenal (HPA) axis function. These effects were measured by determination of plasma cortisol concentrations and responses to adrenocorticotropin challenge (i.e., ACTH stimulation test) in 2 placebo-controlled, 6-week trials in patients with active disease [see Clinical Studies (14)]. These trials enrolled subjects with post-ACTH stimulation cortisol levels of >18 mcg/dL at baseline. Subjects received UCERIS rectal foam 2 mg or a placebo twice daily for 2 weeks followed by once daily for 4 weeks. Normal morning serum cortisol levels >5 mcg/dL were maintained in 85% and 84% of UCERIS rectal foam treated subjects during Weeks 1 and 2 (twice daily treatment) and 93% and 94% during Weeks 4 and 6 (once daily treatment), respectively (see Table 3).
At baseline (predose), 84% of subjects in the UCERIS rectal foam group had a normal response to the ACTH challenge and at Week 6, 63% of subjects had a normal response to the ACTH challenge; in the placebo group, these values were 86% and 76%, respectively (see Table 3). ACTH stimulation test was not performed routinely during the twice daily treatment period (Weeks 1 and 2).
- Table 3: Proportion of Subjects with Normal Endogenous Cortisol Levels (>5 mcg/dL) During the Study and Proportion of Subjects with Normal Response to ACTH Challenge
|
Cortisol Parameter |
UCERIS Rectal Foam
|
Placebo
|
||
|
Total cortisol ˃5 mcg/dL
|
||||
|
Baseline |
259/268 |
(96.6) |
275/278 |
(98.9) |
|
Week 1 |
224/263 |
(85.2) |
264/269 |
(98.1) |
|
Week 2 |
216/257 |
(84.0) |
263/266 |
(98.9) |
|
Week 4 |
218/235 |
(92.8) |
243/249 |
(97.6) |
|
Week 6 |
211/224 |
(94.2) |
234/241 |
(97.1) |
|
Normal response to ACTH challenge |
||||
|
Baseline |
222/266 |
(83.5) |
238/278 |
(85.6) |
|
Week 6 |
148/236 |
(62.7) |
180/237 |
(75.9) |
12.3 Pharmacokinetics
Absorption
Distal Ulcerative Colitis Patients
Based on population pharmacokinetic analysis from sparse PK samples from phase 3 studies,the estimated AUC0-12 following administration of UCERIS rectal foam 2 mg twice a day was 4.31 ng*hr/mL with a CV of 64% in the target patient population.
Distribution
The volume of distribution (VSS) of budesonide varies between 2.2 and 3.9 L/kg in healthy subjects and in patients. Plasma protein binding is estimated to be 85 to 90% in the concentration range of 1 to 230 nmol/L, independent of gender. The erythrocyte/plasma partition ratio at clinically relevant concentrations is approximately 0.8.
Elimination
Metabolism
Following absorption, budesonide is subject to first-pass metabolism. In vitro experiments in human liver microsomes demonstrate that budesonide is rapidly and extensively biotransformed, mainly by CYP3A4, to its 2 major metabolites, 6β-hydroxy budesonide and 16α-hydroxy prednisolone. The glucocorticoid activity of these metabolites is negligible (<1/100) in relation to that of the parent compound.
In vivo investigations with intravenous doses in healthy subjects demonstrate that budesonide has a plasma clearance of 0.9-1.8 L/min. These plasma clearance values approach the estimated liver blood flow, suggesting that budesonide is a high hepatic clearance drug.
Excretion
Budesonide is excreted in urine and feces in the form of metabolites. After oral as well as intravenous administration of micronized [3H]-budesonide, approximately 60% of the recovered radioactivity is found in urine. The major metabolites, including 6β-hydroxybudesonide and 16α-hydroxyprednisolone, are mainly renally excreted, intact or in conjugated forms. No unchanged budesonide is detected in urine.
Specific Populations
Patients with Renal Impairment
The pharmacokinetics of budesonide in patients with renal impairment has not been studied. Intact budesonide is not renally excreted, but metabolites are to a large extent, and might therefore reach higher levels in patients with impaired renal function. However, these metabolites have negligible corticosteroid activity as compared with budesonide.
Patients with Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of UCERIS rectal foam has not been studied. In a study in patients with mild to moderate hepatic impairment (Child-Pugh Class A and Child-Pugh Class B) dosed with budesonide 4 mg oral capsules, systemic exposure was similar between patients with mild hepatic impairment (Child-Pugh Class A; n=4) and healthy subjects (n=8), and 3.5-fold higher in patients with moderate hepatic impairment (Child-Pugh Class B; n=4) than in healthy subjects. For the intravenous dose, no significant differences in CL or VSS are observed. Patients with severe liver dysfunction (Child-Pugh Class C) were not studied [see Use in Specific Populations (8.6)].
Drug Interaction Studies
Budesonide is metabolized via CYP3A4. Potent inhibitors of CYP3A4 can increase the plasma concentrations of budesonide. Co-administration of ketoconazole (inhibitor of CYP3A4) results in an 8-fold increase in AUC of oral budesonide, compared to budesonide alone. Grapefruit juice, an inhibitor of gut mucosal CYP3A, approximately doubles the systemic exposure of oral budesonide. Conversely, induction of CYP3A4 can result in the lowering of budesonide plasma concentrations. The effect of CYP3A4 inhibitors and inducers on the pharmacokinetics of UCERIS rectal foam have not been studied [see Dosage and Administration (2) and Drug Interactions (7)].
Oral contraceptives containing ethinyl estradiol, which are also metabolized by CYP3A4, do not affect the pharmacokinetics of oral budesonide. Budesonide does not affect the plasma concentrations of oral contraceptives (i.e., ethinyl estradiol).
In vitro interaction studies performed with budesonide showed that budesonide did not inhibit human cytochrome P450 isoenzymes CYP1A2, CYP2B6, CYP2C9, CYP2D6, or CYP2E1 at concentrations ranging from 0.11 to 1130 ng/mL. Isoenzyme CYP3A4 was inhibited at the highest concentration tested but the IC50 was >1130 ng/mL. UCERIS rectal foam is not expected to inhibit these enzymes in clinical use. No significant induction of CYP1A2, CYP2B6, CYP2C9 or CYP3A4/5 expression was observed in human hepatocytes in vitro at budesonide concentrations up to 9000 nM (3.88 mcg/mL).
In an in vitro study, budesonide was not a substrate of human transporters OATP1B3 and may be a weak substrate of OATP1B1. Budesonide at concentrations up to 300 nM (129 ng/mL) did not inhibit OATP1B1 or OATP1B3.
Budesonide was not a substrate of BCRP and was a weak substrate of P-glycoprotein. Budesonide was a weak inhibitor of P-glycoprotein (IC50 9.78 µM or 4.21 mcg/mL) and BCRP (IC50 43.1 µM or 18.6 mcg/mL). UCERIS rectal foam is not expected to inhibit these transporters in clinical use.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Carcinogenicity studies with budesonide were conducted in rats and mice. In a 2-year study in Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of gliomas in male rats at an oral dose of 50 mcg/kg (approximately 0.12 times the recommended intrarectal dose of 4 mg/day in humans, based on the body surface area). In addition, there were increased incidences of primary hepatocellular tumors in male rats at 25 mcg/kg (approximately 0.06 times the recommended intrarectal dose of 4 mg/day in humans, based on the body surface area) and above. No tumorigenicity was seen in female rats at oral doses up to 50 mcg/kg (approximately 0.12 times the recommended intrarectal dose of 4 mg/day in humans, based on the body surface area).
In an additional 2-year study in male Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg (approximately 0.12 times the recommended intrarectal dose of 4 mg/day in humans, based on the body surface area). However, it caused a statistically significant increase in the incidence of hepatocellular tumors at an oral dose of 50 mcg/kg (approximately 0.12 times the recommended intrarectal dose of 4 mg/day in humans, based on the body surface area). The concurrent reference glucocorticosteroids (prednisolone and triamcinolone acetonide) showed similar findings. In a 91-week study in mice, budesonide caused no treatment-related carcinogenicity at oral doses up to 200 mcg/kg (approximately 0.24 times the recommended intrarectal dose of 4 mg/day in humans, based on the body surface area).
Mutagenesis
Budesonide showed no evidence of mutagenic potential in the Ames test, the mouse lymphoma cell forward gene mutation (TK+/-) test, the human lymphocyte chromosome aberration test, the Drosophila melanogaster sex-linked recessive lethality test, the rat hepatocyte UDS test or the mouse micronucleus test.
Impairment of Fertility
In rats, budesonide had no effect on fertility at subcutaneous doses up to 80 mcg/kg (approximately 0.20 times recommended intrarectal dose of 4 mg/day in humans, based on the body surface area). However, it caused a decrease in prenatal viability and viability in pups at birth and during lactation, along with a decrease in maternal body-weight gain, at subcutaneous doses of 20 mcg/kg (approximately 0.05 times recommended intrarectal dose of 4 mg/day in humans, based on the body surface area) and above. No such effects were noted at 5 mcg/kg.
14 CLINICAL STUDIES
The safety and efficacy of UCERIS rectal foam were evaluated in 2 replicate, randomized, double-blind, placebo-controlled, multi-center trials (Studies 1 and 2).
Participants in the trials were adult patients with active mild-to-moderate distal ulcerative colitis with disease extending at least 5 cm but no further than 40 cm from the anal verge (confirmed by endoscopy). To be eligible, patients had to have a Modified Mayo Disease Activity Index (MMDAI) score between 5 and 10, inclusive, a rectal bleeding subscore of 2 or 3, and an endoscopy subscore of 2 or 3. The MMDAI score ranges from 0 to 12 and has 4 subscales that are each scored from 0 (normal) to 3 (most severe): stool frequency, rectal bleeding, findings on endoscopy, and physician global assessment. An endoscopy subscore of 2 is defined by marked erythema, lack of vascular pattern, friability, and erosions; an endoscopy subscore of 3 is defined by spontaneous bleeding and ulceration.
Oral and rectal corticosteroids, and rectal 5-aminosalicylic acid (5-ASA) products were prohibited during the course of the trials but were allowed as rescue therapy. Oral 5-ASA products were allowed at doses ≤ 4.8 grams/day.
In total, 546 subjects were randomized in these trials: 267 subjects to UCERIS rectal foam and 279 subjects to placebo. In each trial (Study 1 and Study 2), patients received UCERIS rectal foam 2 mg or placebo twice daily for 2 weeks followed by once daily for 4 weeks.
The median age was 41 years and 42 years, 5% and 8% were ≥ 65 years of age, and 43% and 45% were male, in Studies 1 and 2, respectively. In each of these trials, 90% were Caucasian, 7-8% were African American, and 3% were Asian or Other.
The majority of patients had a baseline diagnosis of proctosigmoiditis (69% and 74%) in Studies 1 and 2, respectively. The remaining patients had a baseline diagnosis of proctitis. Concomitant oral 5-ASA use at baseline was 59% and 51% in Studies 1 and 2, respectively.
Baseline MMDAI total score was 7.8 and 7.9 in the UCERIS rectal foam group and placebo group, respectively, of Study 1; and 7.9 and 8.0 in the UCERIS rectal foam group and placebo group, respectively, of Study 2. The mean stool frequency subscore at baseline was 1.8 and 1.9 in the UCERIS rectal foam group and placebo group, respectively, of Study 1; and 1.7 and 1.8 in the UCERIS rectal foam group and placebo group, respectively, of Study 2.
In each trial (Study 1 and Study 2), the primary endpoint was the proportion of subjects who were in remission after 6 weeks of treatment. Remission was defined as a decrease or no change in the stool frequency subscore from baseline, a rectal bleeding subscore of 0, and an endoscopy score of 0 or 1. (An endoscopy subscore of zero is defined by normal or inactive disease; an endoscopy subscore of 1 is defined by erythema and decreased vascular pattern.)
In each trial (Study 1 and Study 2), a higher proportion of patients in the UCERIS rectal foam group than in the placebo group were in remission at Week 6 and had a rectal bleeding subscore of 0 at Week 6 (Table 4).
|
Study 1 |
||||
|
Efficacy Endpoint |
UCERIS Rectal Foam
|
Placebo
|
p-value |
Treatment Difference
|
|
Remission at Week 6 |
38.3% |
25.8% |
0.032 |
12.6% |
|
Rectal Bleeding subscore |
46.6% |
28.0% |
0.002 |
18.6% |
|
Study 2 |
||||
|
UCERIS Rectal Foam
|
Placebo
|
p-value |
Treatment Difference
|
|
|
Remission at Week 6 |
44.0% |
22.4% |
<0.001 |
21.6% |
|
Rectal Bleeding subscore |
50.0% |
28.6% |
<0.001 |
21.4% |
CI: Confidence Interval
In Study 1, the percentage of patients with endoscopy subscore of 0 or 1 at Week 6 was 55.6% in the UCERIS rectal foam group versus 43.2% in the placebo group. In Study 2, the percentage of patients with endoscopy subscore of 0 or 1 at Week 6 was 56.0% in the UCERIS rectal foam group versus 36.7% in the placebo group (an endoscopy subscore of 0 is defined by normal or inactive disease; an endoscopy subscore of 1 is defined by erythema and decreased vascular pattern).
In patients that met the primary endpoint of remission in Study 1, the mean (SD) decrease in stool frequency subscore was 1.2 (0.9) in the UCERIS rectal foam group and 1.2 (0.8) in the placebo group. In patients that met the primary endpoint of remission in Study 2, the mean (SD) decrease in stool frequency subscore was 1.3 (0.8) in the UCERIS rectal foam group and 1.1 (0.9) in the placebo group.
16 HOW SUPPLIED/STORAGE AND HANDLING
UCERIS rectal foam is supplied as a kit containing 2 aerosol canisters with 28 PVC applicators coated with paraffin lubricant for administration of the foam (NDC 65649-651-03). Each canister (NDC 65649-651-02) is labeled with a net weight of 33.4 g and contains 14 metered doses.
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Handling
UCERIS rectal foam contains a flammable propellant. Do not have the canister burned after use and do not spray contents directly towards flames.
- •
- Do not expose to heat or store at temperatures above 120°F (49°C).
- •
- Flammable. Avoid fire, flame, or smoking during and immediately following administration.
- •
- Contents under pressure. Do not puncture or incinerate.
DO NOT REFRIGERATE.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Administration
Advise patients:
- •
- UCERIS rectal foam is only to be applied rectally. It is not for oral use.
- •
- Before using UCERIS rectal foam, use the bathroom to empty your bowels.
- •
- Each applicator is coated with a lubricant. If additional lubrication is needed, petrolatum or petroleum jelly can also be used.
- •
- Warm the canister in the hands while shaking it vigorously for 10 to 15 seconds prior to use.
- •
- UCERIS rectal foam can be used in a standing, lying or sitting position (e.g., while using the toilet).
- •
- Apply UCERIS rectal foam in the morning and the evening for the first 2 weeks of treatment; then once daily in the evening for the next 4 weeks. When applied in the evening, use immediately prior to bedtime. Try not to empty your bowels again until the next morning.
- •
- Avoid consumption of grapefruit or grapefruit juice during treatment with UCERIS rectal foam.
- •
- Avoid fire, flame, and smoking during and immediately following administration since UCERIS rectal foam is flammable.
Hypercorticism and Adrenal Suppression
Advise patients that UCERIS rectal foam may cause hypercorticism and adrenal suppression and that they should taper slowly from systemic corticosteroids if transferring to UCERIS rectal foam [see Warnings and Precautions (5.1), (5.2)]. Advise patients that replacement of systemic glucocorticosteroids with UCERIS rectal foam may unmask allergies (e.g., rhinitis and eczema), which were previously controlled by the systemic drug.
Increased Risk of Infection
Advise patients to avoid exposure to people with chicken pox or measles and if exposed, consult a physician. Also, inform patients that they are at increased risk of developing a variety of infections, including worsening of existing tuberculosis, fungal, bacterial, viral, or parasitic infections, or ocular herpes simplex and to contact their physician if they develop any symptoms of infection [see Warnings and Precautions (5.3)].
Pregnancy
Advise female patients that UCERIS rectal foam may cause fetal harm and to inform their healthcare provider with a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Distributed by:
Salix Pharmaceuticals, a division of
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
ASM Aerosol-Service AG
Industriestrasse 11
CH-4313 Möhlin Switzerland
Product marketed and distributed under license from Dr. Falk Pharma.
UCERIS is a trademark of Salix Pharmaceuticals, Inc. or its affiliates.
© 2020 Salix Pharmaceuticals, Inc. or its affiliates 9614101
EX00152
Patient Information
UCERIS® (u SAIR us)
(budesonide)
rectal foam
What is UCERIS rectal foam?
UCERIS rectal foam is a prescription corticosteroid medicine used to help get mild to moderate active ulcerative colitis that extends from the rectum to the sigmoid colon under control (induce remission).
It is not known if UCERIS rectal foam is safe and effective in children.
Who should not use UCERIS rectal foam?
Do not use UCERIS rectal foam if you are allergic to budesonide or any of the ingredients in UCERIS rectal foam. See the end of this leaflet for a complete list of ingredients in UCERIS rectal foam.
What should I tell my healthcare provider before using UCERIS rectal foam?
Before you use UCERIS rectal foam, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have liver problems.
- •
- are planning to have surgery.
- •
- have chicken pox or measles or have recently been near anyone with chicken pox or measles.
- •
- have an infection.
- •
- have or had a family history of diabetes, cataracts or glaucoma.
- •
- have or had tuberculosis.
- •
- have high blood pressure (hypertension).
- •
- have decreased bone mineral density (osteoporosis).
- •
- have stomach ulcers.
- •
- are pregnant or plan to become pregnant. It is not known if UCERIS rectal foam may harm your unborn baby. Tell your healthcare provider if you are pregnant or think you are pregnant.
- •
- are breastfeeding or plan to breastfeed. UCERIS rectal foam can pass into your breast milk and may harm your baby. You and your healthcare provider should decide if you will use UCERIS rectal foam or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. UCERIS rectal foam and other medicines may affect each other causing side effects. Especially tell your healthcare provider if you take another medicine that contains corticosteroids for other conditions, such as allergies or asthma.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use UCERIS rectal foam?
See the “Instructions for Use” at the end of this Patient Information for detailed information about the right way to use UCERIS rectal foam.
- •
- Use UCERIS rectal foam exactly as your healthcare provider tells you to use it.
- •
- UCERIS rectal foam should only be used rectally (through the anus).Do not take UCERIS rectal foam by mouth.
- •
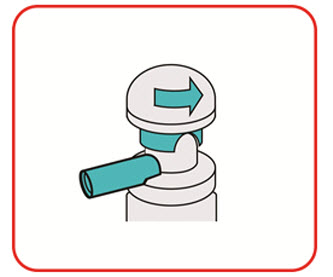
- Warm the UCERIS rectal foam canister by holding it in your hands while shaking it (vigorously) for 10 to 15 seconds.
- •
- UCERIS rectal foam is used twice a day for the first 2 weeks of treatment (in the morning and in the evening). After 2 weeks, use UCERIS rectal foam 1 time a day in the evening, before bedtime for 4 weeks.
- •
- If you use too much UCERIS rectal foam, call your healthcare provider right away.
- •
- You should stop using UCERIS rectal foam before preparing for a colonoscopy. Call your healthcare provider before restarting UCERIS rectal foam after your colonoscopy.
What should I avoid while using UCERIS rectal foam?
- •
- Do not eat grapefruit or drink grapefruit juice while using UCERIS rectal foam. Eating grapefruit or drinking grapefruit juice can increase the level of UCERIS rectal foam in your blood.
- •
- UCERIS rectal foam is flammable. Avoid fire, flame and smoking during and right after using UCERIS rectal foam.
What are the possible side effects of UCERIS rectal foam?
UCERIS rectal foam may cause serious side effects, including:
- •
- Effects of having too much corticosteroid medicine in your blood (hypercorticism). Long-time use of UCERIS rectal foam can cause you to have too much glucocorticosteroid medicine in your blood. Tell your healthcare provider if you have any of the following signs and symptoms of hypercorticism:
- •
- acne
- •
- bruise easily
- •
- rounding of your face (moon face)
- •
- ankle swelling
- •
- thicker or more hair on your body and face
- •
- a fatty pad or hump between your shoulders (buffalo hump)
- •
- pink or purple stretch marks on the skin of your abdomen, thighs, breasts and arms
- •
-
Adrenal suppression. When UCERIS rectal foam is used for a long period of time (chronic use), the adrenal glands may not make enough steroid hormones (adrenal suppression). Tell your healthcare provider if you are under stress or have any symptoms of adrenal suppression during treatment with UCERIS rectal foam including:
- •
- tiredness
- •
- weakness
- •
- nausea
- •
- vomiting
- •
- low blood pressure
- •
- Immune system effects and a higher chance of infections. UCERIS rectal foam may weaken your immune system. Taking medicines that weaken your immune system makes you more likely to get infections. Avoid contact with people who have contagious diseases such as chicken pox or measles while using UCERIS rectal foam.
- Tell your healthcare provider about any signs or symptoms of infection during treatment with UCERIS rectal foam, including:
|
|
|
|
|
|
- •
- Worsening of allergies. If you take certain other corticosteroid medicines to treat allergies, switching to UCERIS rectal foam may cause your allergies to come back. These allergies may include eczema (a skin disease) or inflammation inside your nose (rhinitis). Tell your healthcare provider if any of your allergies become worse while using UCERIS rectal foam.
The most common side effects of UCERIS rectal foam include:
- •
- decreased blood cortisol levels
- •
- adrenal insufficiency
- •
- nausea
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of UCERIS rectal foam. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store UCERIS rectal foam?
- •
- Store UCERIS rectal foam at room temperature, between 68° to 77°F (20° to 25°C).
- •
- Do not store the UCERIS rectal foam container near heat or store at temperatures above 120°F (49°C).
- •
- Do not puncture or burn the UCERIS rectal foam canister.
- •
- Do not refrigerate.
Keep UCERIS rectal foam and all medicines out of reach of children.
General information about the safe and effective use of UCERIS rectal foam.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use UCERIS rectal foam for a condition for which it was not prescribed. Do not give UCERIS rectal foam to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about UCERIS rectal foam. If you would like more information, talk with your healthcare provider. You may ask your healthcare provider or pharmacist for information about UCERIS rectal foam that is written for health professionals.
For more information, go to www.ucerisfoam.com
What are the ingredients in UCERIS rectal foam?
Active ingredient: budesonide
Inactive ingredients: cetyl alcohol, citric acid monohydrate, edetate disodium, emulsifying wax, polyoxyl (10) stearyl ether, propylene glycol, and purified water
Propellant: n-butane, isobutane, and propane
Distributed by:
Salix Pharmaceuticals, a division of
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
ASM Aerosol-Service AG
Industriestrasse 11
CH-4313 Möhlin Switzerland
Product marketed and distributed under license from Dr. Falk Pharma.
UCERIS is a trademark of Salix Pharmaceuticals, Inc. or its affiliates.
© 2020 Salix Pharmaceuticals, Inc. or its affiliates
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 04/2020
9614101 EX00152
Instructions for Use
UCERIS® (u SAIR us)
(budesonide)
rectal foam
Read the Patient Information and Instructions for Use that comes with UCERIS rectal foam before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. Talk to your healthcare provider if you have any questions.
Before using UCERIS rectal foam, you should use the bathroom to empty your bowels.
You may use UCERIS rectal foam while in a standing position, in a lying position, or in a sitting position (for example, while using the toilet).
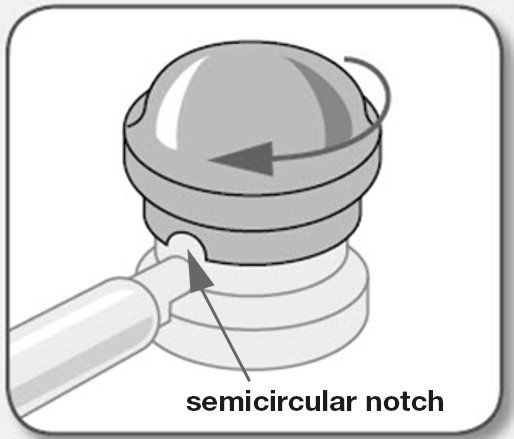
Applicators should be used only 1 time. You should use a new applicator for each dose.
Figure A
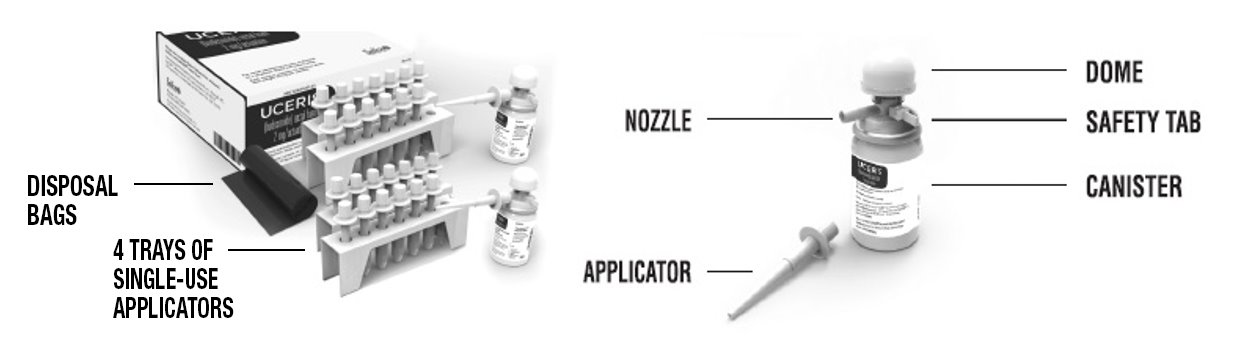
Each kit contains (See Figure A):
- •
- Complete Prescribing Information
- •
- Patient Information and Instructions for Use
- •
- 2 canisters containing 14 doses each
- •
- 4 trays of single-use applicators (7 applicators per tray)
- •
- Applicator throw away (disposal) bags for use after each dose
Preparing to use UCERIS rectal foam
Distributed by:
Salix Pharmaceuticals, a division of
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
ASM Aerosol-Service AG
Industriestrasse 11
CH-4313 Möhlin Switzerland
Product marketed and distributed under license from Dr. Falk Pharma.
UCERIS is a trademark of Salix Pharmaceuticals, Inc. or its affiliates.
© 2020 Salix Pharmaceuticals, Inc. or its affiliates
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 04/2020 9614101
EX00152