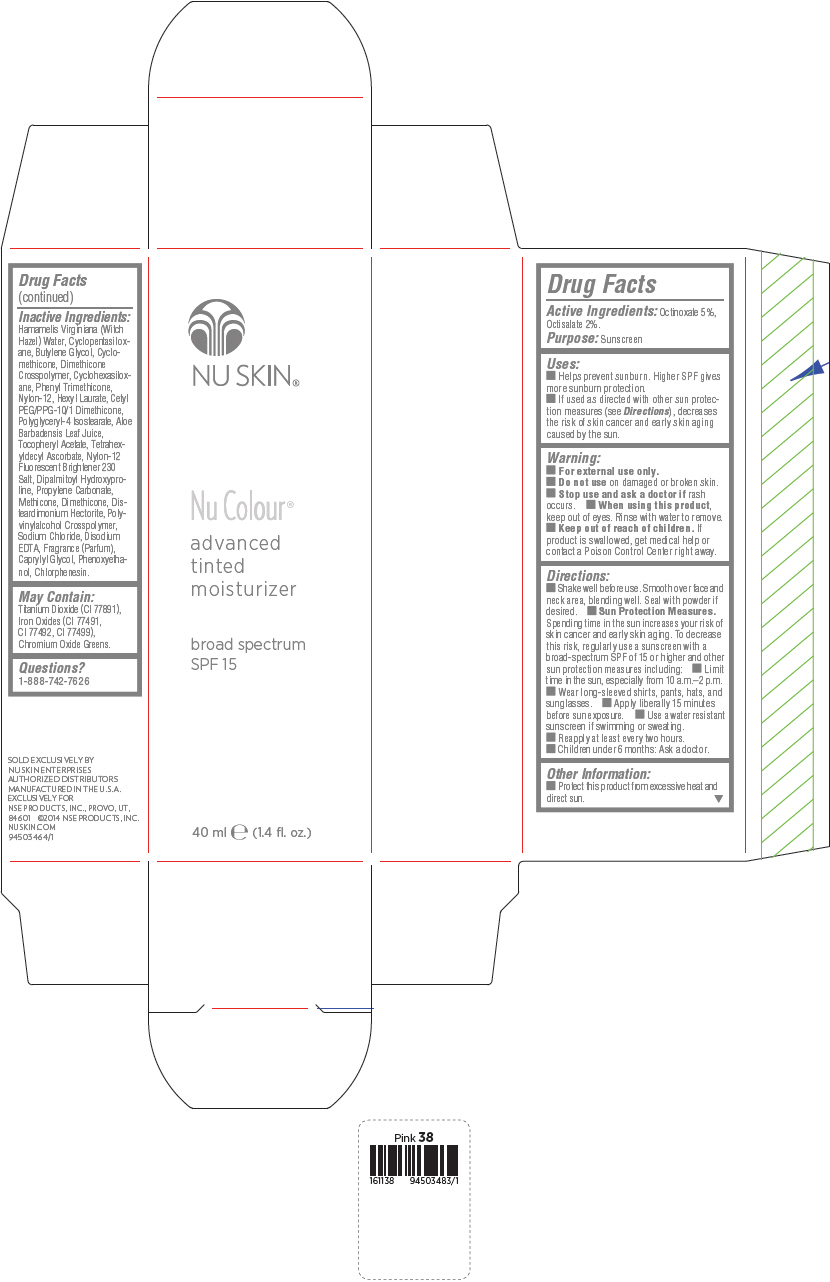
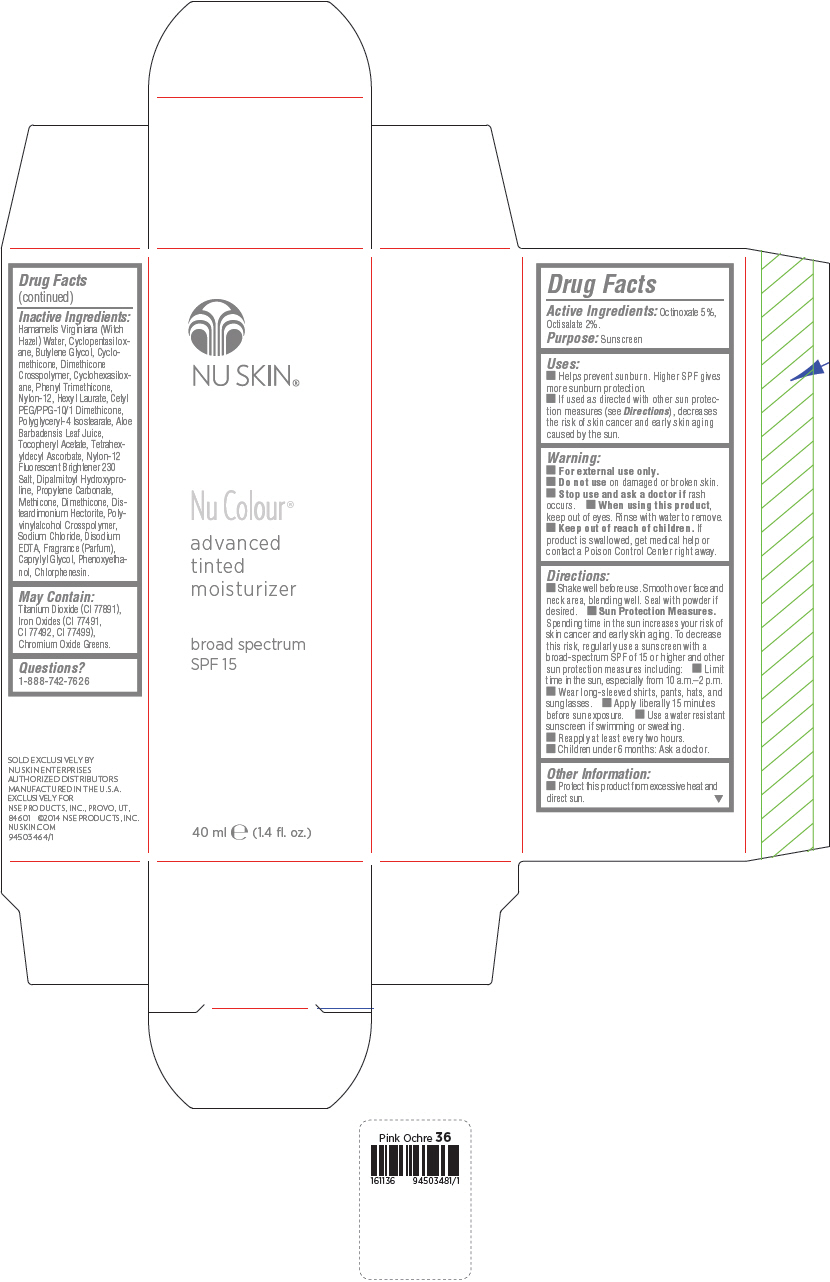
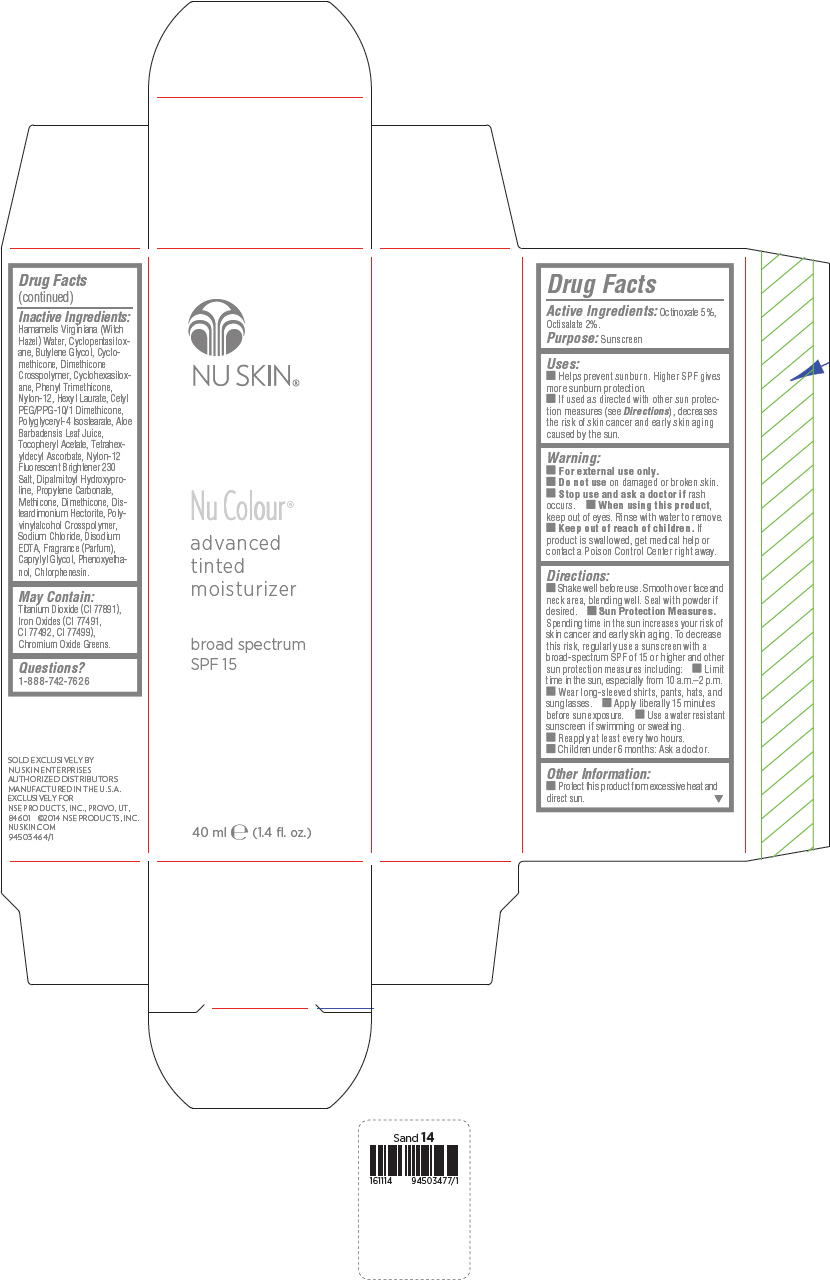
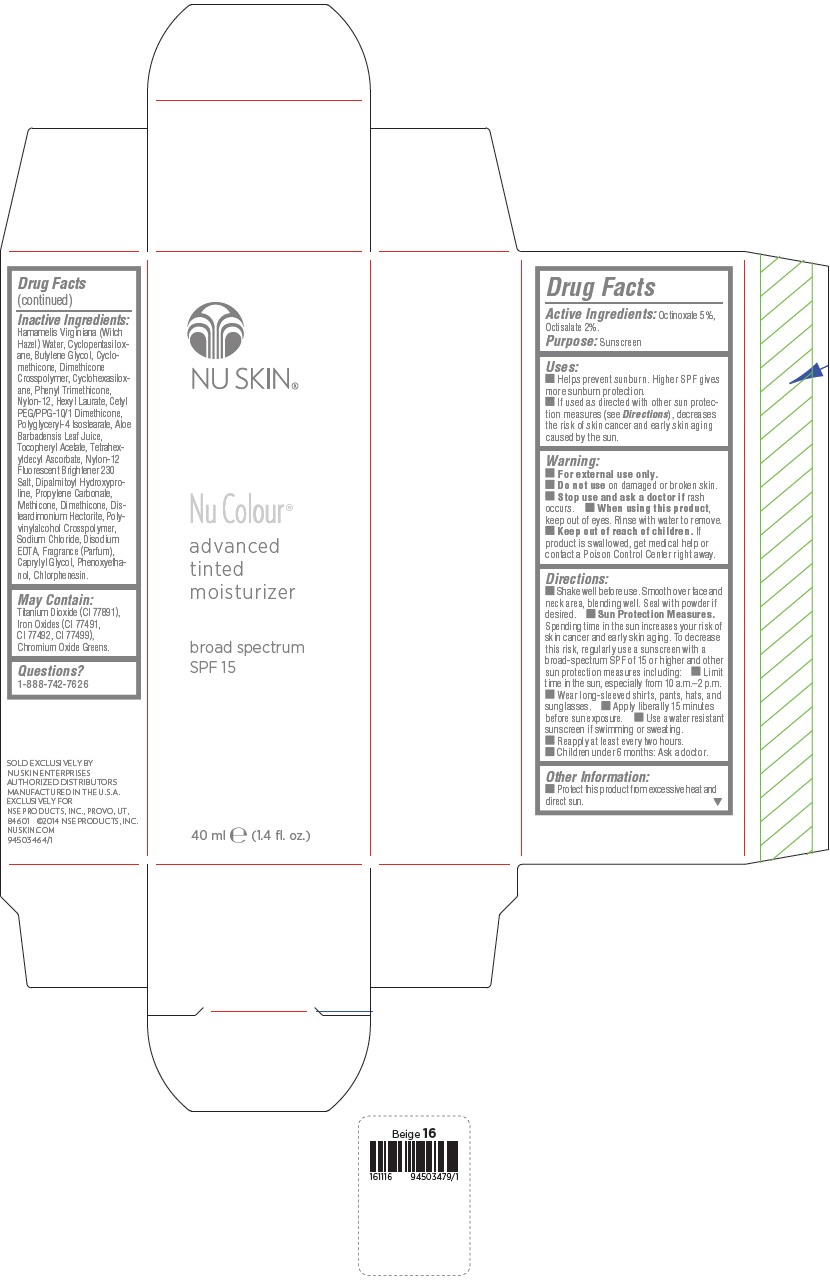
Uses
- Helps prevent sunburn. Higher SPF gives more sunburn protection.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Shake well before use. Smooth over face and neck area, blending well. Seal with powder if desired.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every two hours.
- Children under 6 months: Ask a doctor.
Inactive Ingredients
Hamamelis Virginiana (Witch Hazel) Water, Cyclopentasiloxane, Butylene Glycol, Cyclomethicone, Dimethicone Crosspolymer, Cyclohexasiloxane, Phenyl Trimethicone, Nylon-12, Hexyl Laurate, Cetyl PEG/PPG-10/1 Dimethicone, Polyglyceryl-4 Isostearate, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate, Tetrahexyldecyl Ascorbate, Nylon-12 Fluorescent Brightener 230 Salt, Dipalmitoyl Hydroxyproline, Propylene Carbonate, Methicone, Dimethicone, Disteardimonium Hectorite, Polyvinylalcohol Crosspolymer, Sodium Chloride, Disodium EDTA, Fragrance (Parfum), Caprylyl Glycol, Phenoxyethanol, Chlorphenesin.
May Contain:
Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499), Chromium Oxide Greens.
PRINCIPAL DISPLAY PANEL - 40 mL Bottle Carton - Beige
NU SKIN®
Nu Colour®
advanced
tinted
moisturizer
broad spectrum
SPF 15
40 ml e (1.4 fl. oz.)
PRINCIPAL DISPLAY PANEL - 40 mL Bottle Carton - Fair
NU SKIN®
Nu Colour®
advanced
tinted
moisturizer
broad spectrum
SPF 15
40 ml e (1.4 fl. oz.)
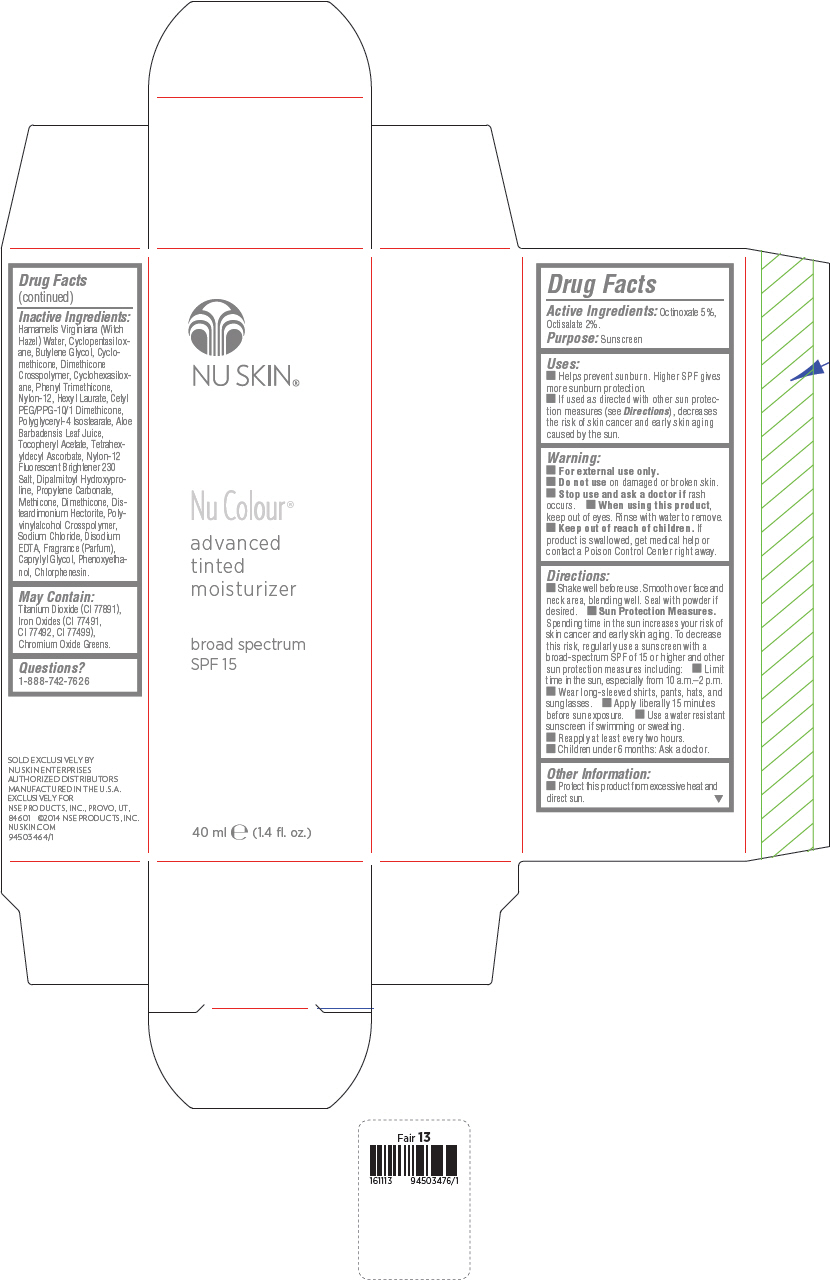
PRINCIPAL DISPLAY PANEL - 40 mL Bottle Carton - Honey
NU SKIN®
Nu Colour®
advanced
tinted
moisturizer
broad spectrum
SPF 15
40 ml e (1.4 fl. oz.)
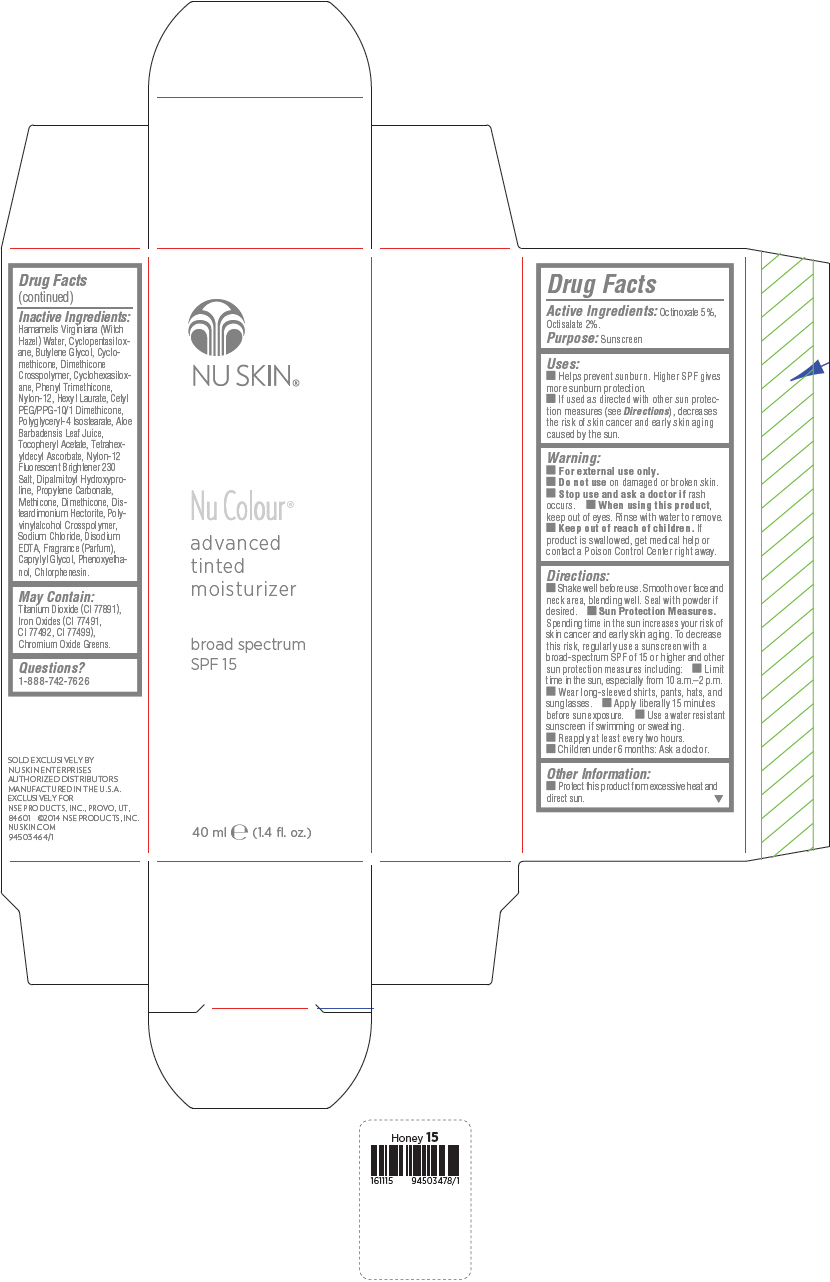
PRINCIPAL DISPLAY PANEL - 40 mL Bottle Carton - Medium Beige
NU SKIN®
Nu Colour®
advanced
tinted
moisturizer
broad spectrum
SPF 15
40 ml e (1.4 fl. oz.)
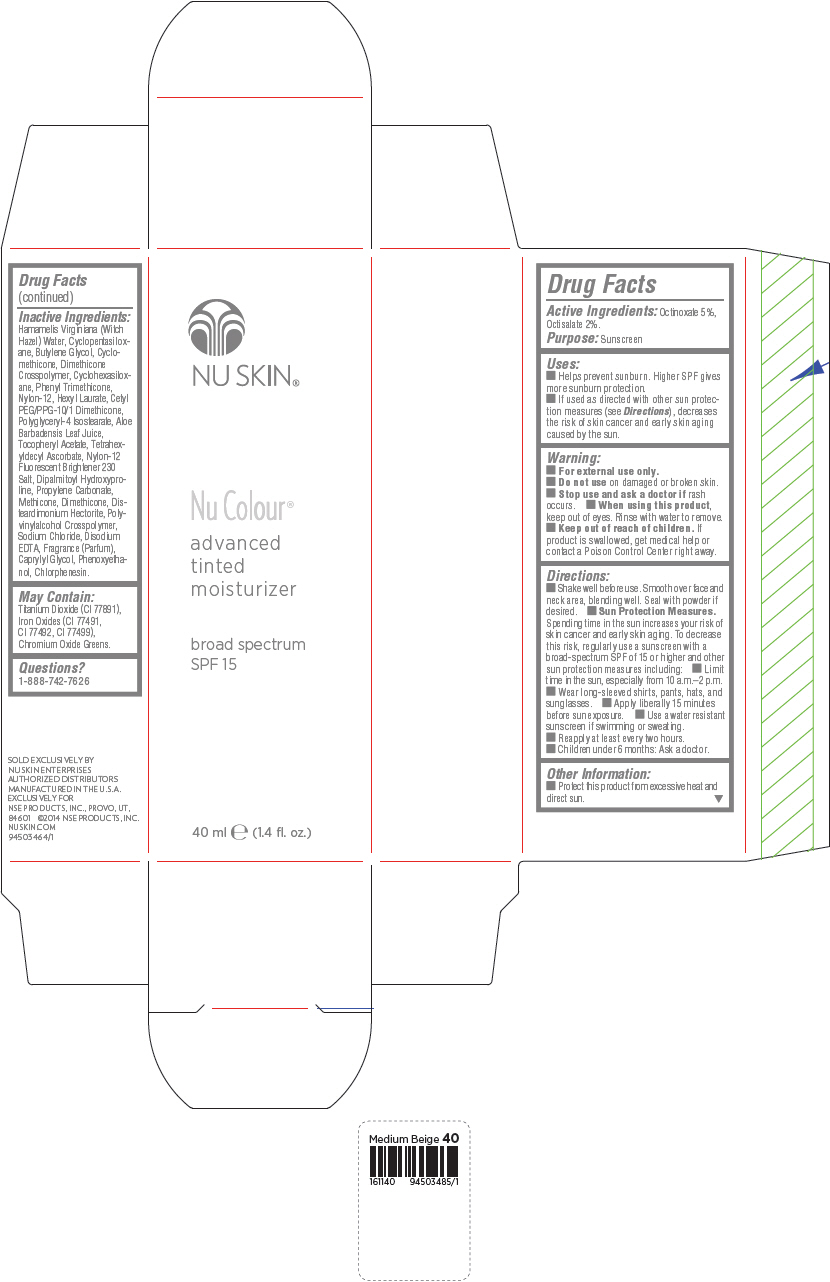
PRINCIPAL DISPLAY PANEL - 40 mL Bottle Carton - Medium Ochre
NU SKIN®
Nu Colour®
advanced
tinted
moisturizer
broad spectrum
SPF 15
40 ml e (1.4 fl. oz.)
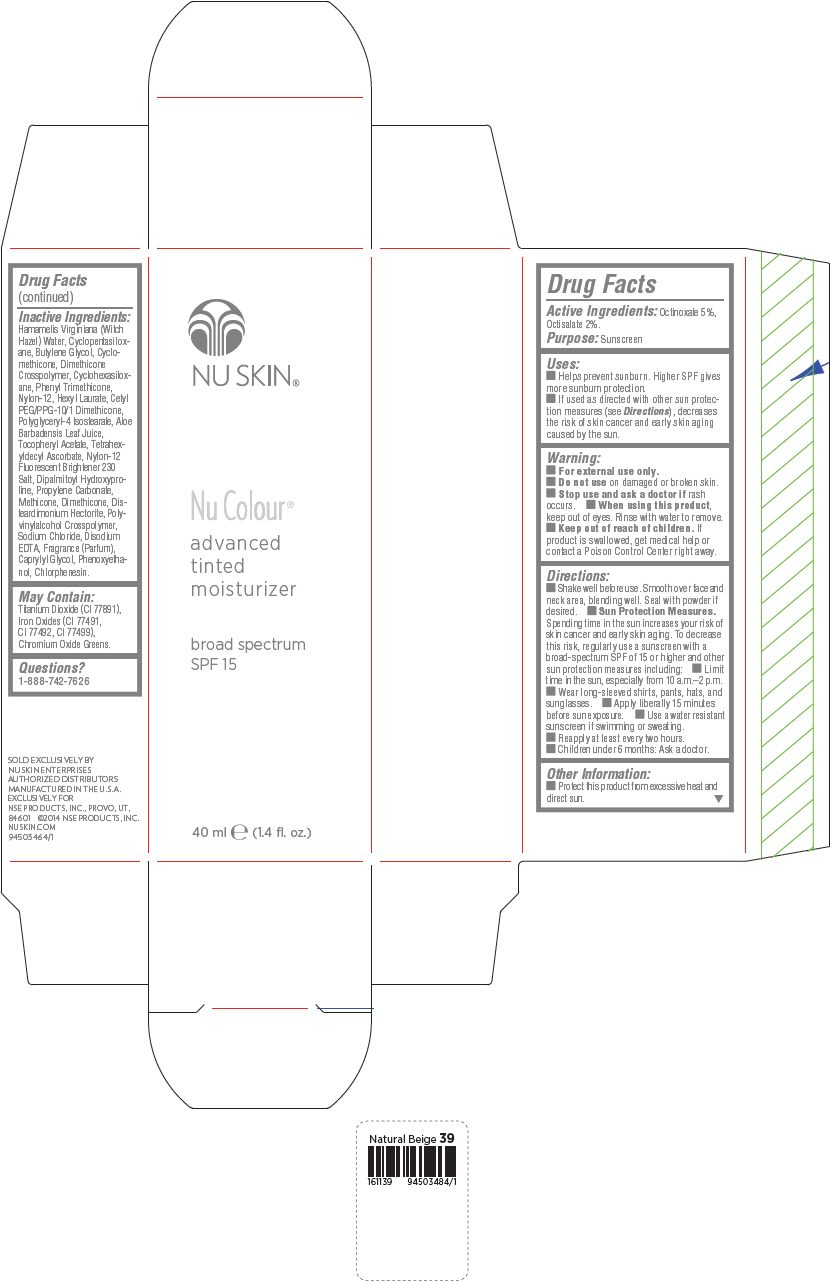
PRINCIPAL DISPLAY PANEL - 40 mL Bottle Carton - Natural Beige
NU SKIN®
Nu Colour®
advanced
tinted
moisturizer
broad spectrum
SPF 15
40 ml e (1.4 fl. oz.)
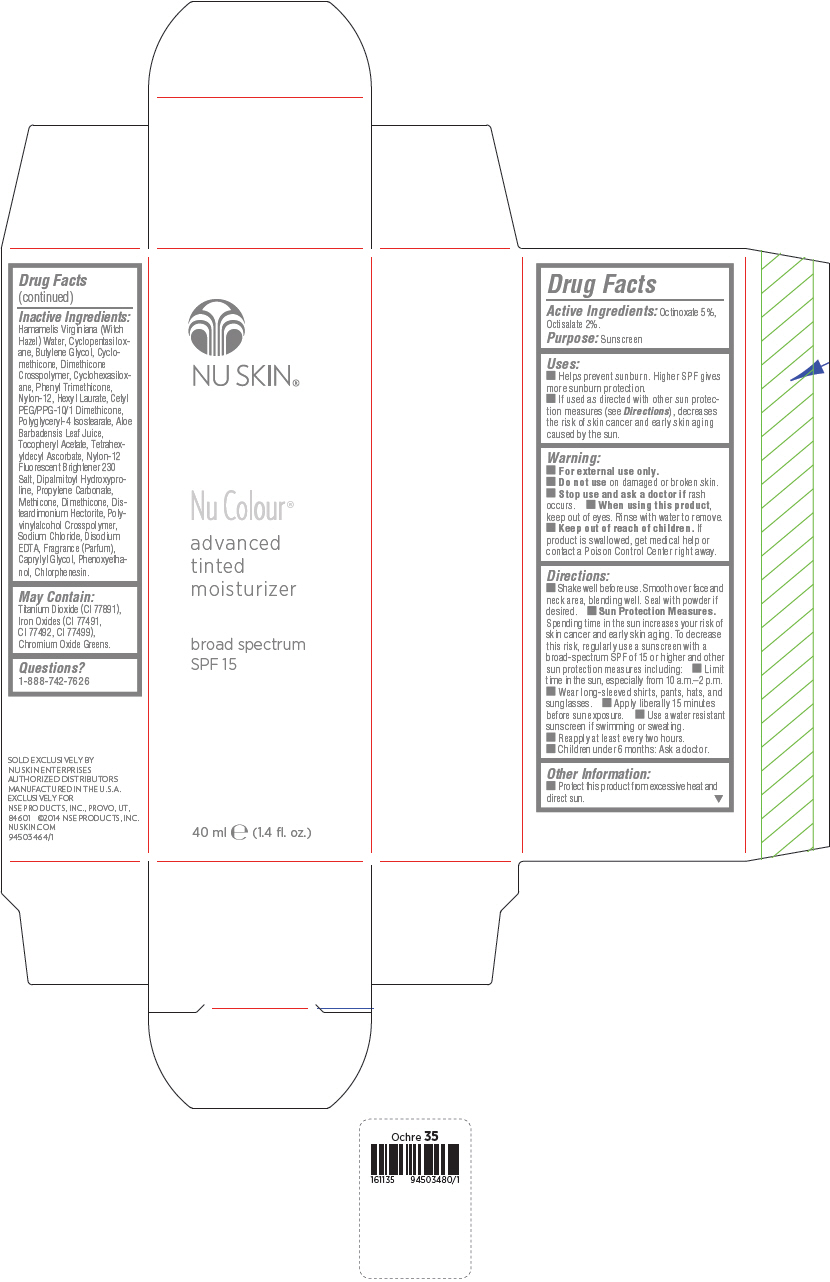
PRINCIPAL DISPLAY PANEL - 40 mL Bottle Carton - Ochre
NU SKIN®
Nu Colour®
advanced
tinted
moisturizer
broad spectrum
SPF 15
40 ml e (1.4 fl. oz.)
PRINCIPAL DISPLAY PANEL - 40 mL Bottle Carton - Pink
NU SKIN®
Nu Colour®
advanced
tinted
moisturizer
broad spectrum
SPF 15
40 ml e (1.4 fl. oz.)