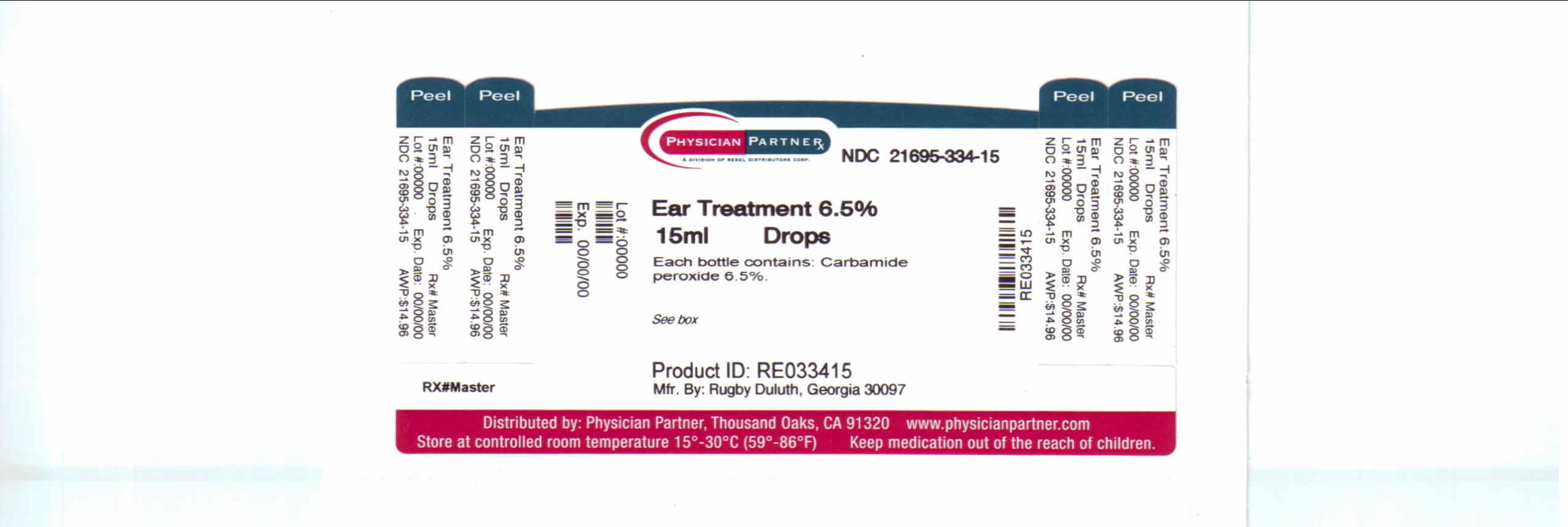
EAR WAX TREATMENT- carbamide peroxide solution/ drops
Rebel Distributors Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Carbamide peroxide 6.5%
Purpose
Earwax removal aid
Use
softens, loosens and removes excessive earwax
Warnings
Do not use for more than 4 days
Ask a doctor before use if you have
- ear drainage or discharge
- ear pain, irritation, rash or are dizzy
- an injury or perforation (hole) of the eardrum or if you had ear surgery
When using this product avoid contact with the eyes
Stop use and ask a doctor if earwax remains longer than 4 days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
FOR USE IN THE EAR ONLY
adults and children over 12 years:
- tilt head sideways and place 5 to 10 drops into ear
- tip of applicator should not enter ear canal
- keep drops in ear for several minutes by tilting head or placing cotton in ear
- use twice daily for up to 4 days
- remove any wax remaining after treatment by gently flushing ear with warm water using a soft rubber bulb ear syringe
children under 12 years: ask a doctor
Other information
- store at room temperature 15° - 30°C (59° - 89°F)
- keep in a dry place
Inactive ingredients
citric acid (anhydrous), glycerin, propylene glycol, purified water, sodium acid pyrophosphate, sodium citrate (anhydrous), trolamine
Questions or comments?
Call 1-800-645-2158
9am - 5pm ET
Monday - Friday
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
PRINCIPAL DISPLAY PANEL - 15 mL Carton
COMPARE TO
ACTIVE INGREDIENT IN
DEBROX®*
Rugby®
NDC 21695-334-15
Earwax
Treatment
Drops
Carbamide Peroxide 6.5 %
Helps Remove
Excessive Earwax
1/2 fl oz (15 mL)
SATISFACTION GUARANTEED
Rugby
OR YOUR MONEY BACK