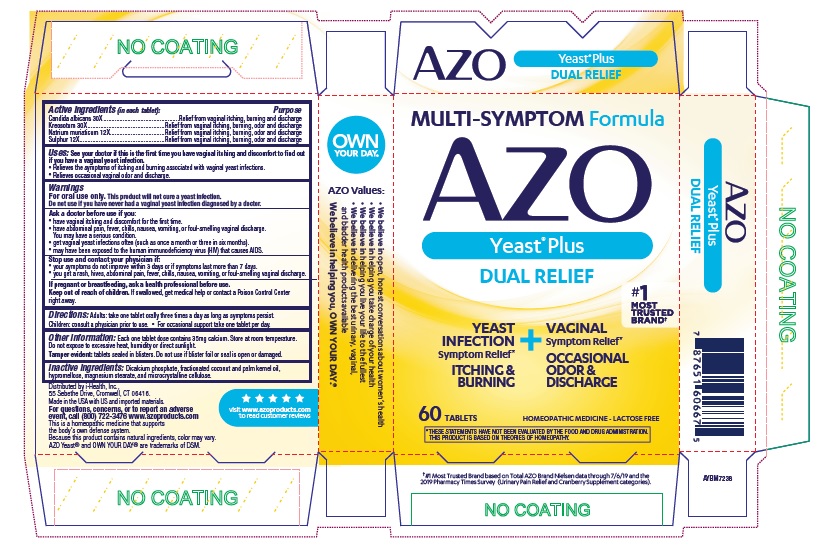
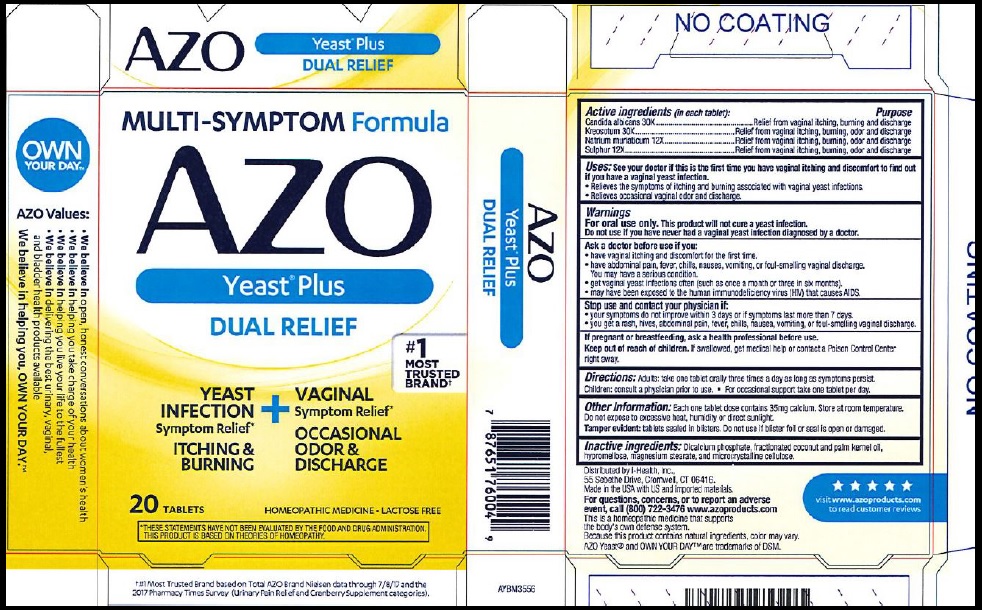
Active Ingredients (in each tablet):
Candida albicans30X
Kreosotum30X
Natrium muriaticum12X
Sulphur12X
Purpose
Relief from vaginal itching, burning and discharge
Relief from vaginal itching, burning, odor and discharge
Relief from vaginal itching, burning, odor and discharge
Relief from vaginal itching, burning, odor and discharge
Use:
See your doctor if this is the first time you have vaginal itching and discomfort to find out if you have a vaginal yeast infection.
- Relieves the symptoms of itching and burning associated with vaginal yeast infections.
- Relieves occasional vaginal odor and discharge.
Warnings:
For oral use only. This product will not cure a yeast infection.
Do not use if you have never had a vaginal yeast infection diagnosed by a doctor.
Ask a doctor before use if you
- have vaginal itching and discomfort for the first time.
- have abdominal pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a serious condition.
- get vaginal yeast infections often (such as once a month or three in six months).
- may have been exposed to human immunodeficiency virus (HIV) that causes AIDS.
Directions: Adults: take one tablet orally three times a day as long as symptoms persist. Children: consult a physician prior to use.
- For occasional support take one tablet per day.
OtherInformation: Each one tablet dose contains 35 mg calcium. Store at room temperature. Do not expose to excessive heat, humidity or direct sunlight.
- Tamper evident: tablets sealed in blisters. Do not use if blister foil or seal is open or damaged.
Inactive Ingredients: Dicalcium phosphate, fractionated cocunut and palm kernel oil, hypromellose, magnesium stearate, and microcrystalline cellulose.
This is a homeopathic medicine that supports the body's own defense system. Because this product contains natural ingredients, color may vary.