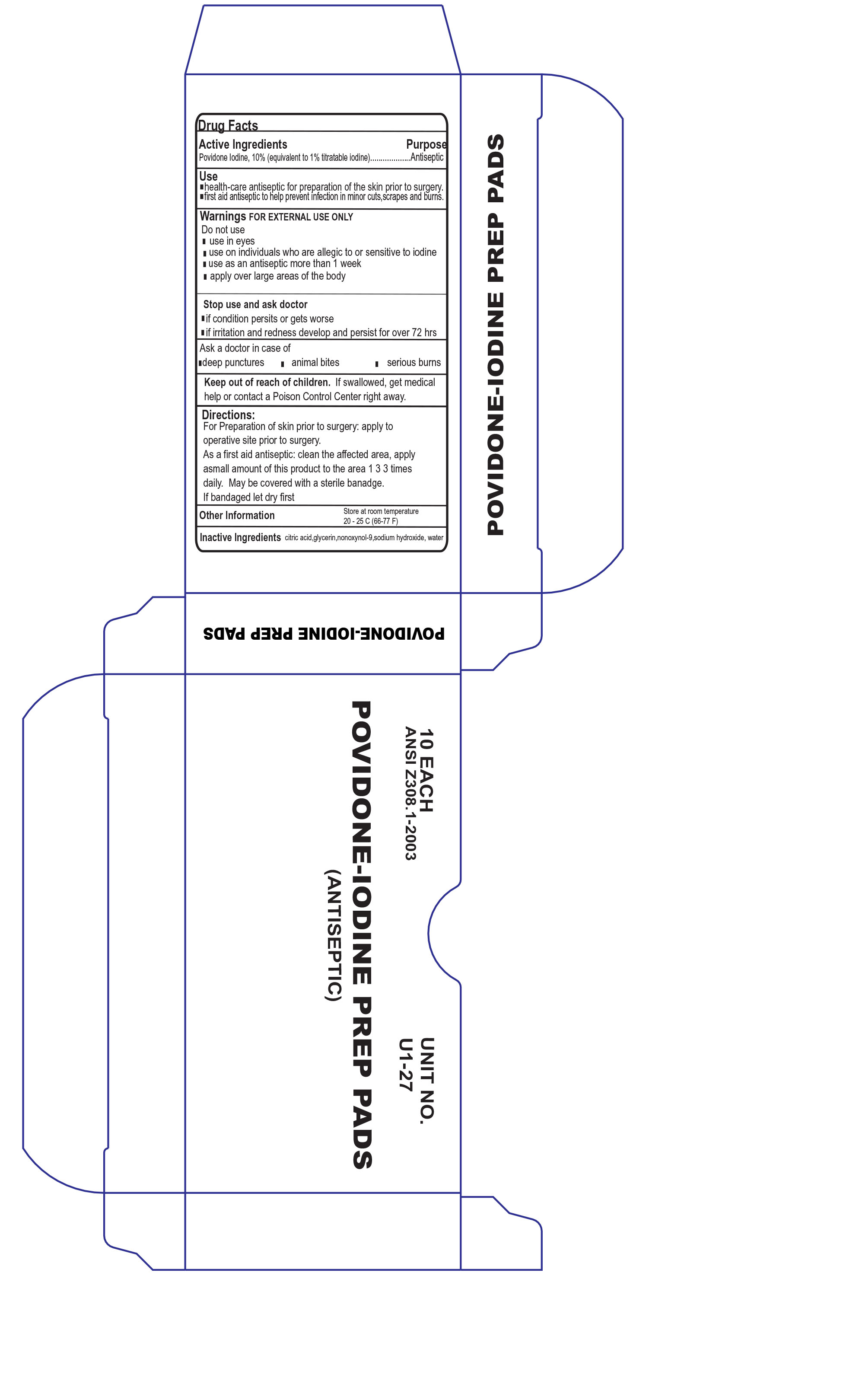
POVIDONE IODINE PREP PADS- povidone iodine prep pads cloth
Custom Kits Company Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Povidone Iodine 10%
Uses
Antiseptic cleanser to help prevent skin infection in minor cuts, scrapes and burns.
For preparation of the skin prior to surgery
Helps reduct bacteria that can potentially cause skin infection
Warnings
For External Use Only
Do Not Use
In the eyes
As a first aid antiseptic for more than 1 week
Over large areas of the body
Ask doctor before use if you have
Deep or puncuture wounds
Animal bites
Serious wounds
Stop Use and ask doctor if
Condition worsens or persists for more than 72 hours
Irritation and rednes develops
Keep Out of the reach of Children
If swallowed, get medical help or contact a Poson Control Center immediately
Directions
Tear at notch
Remove applicator
Use only once
As a first aid antiseptic - Clean affected area
apply 1 to 3 times daily
May be covered with a sterile bandage
If bandaged let dry first
Store at room temperature
Avoid excessive heat
Inactive ingredient
nonoxynol-9, water