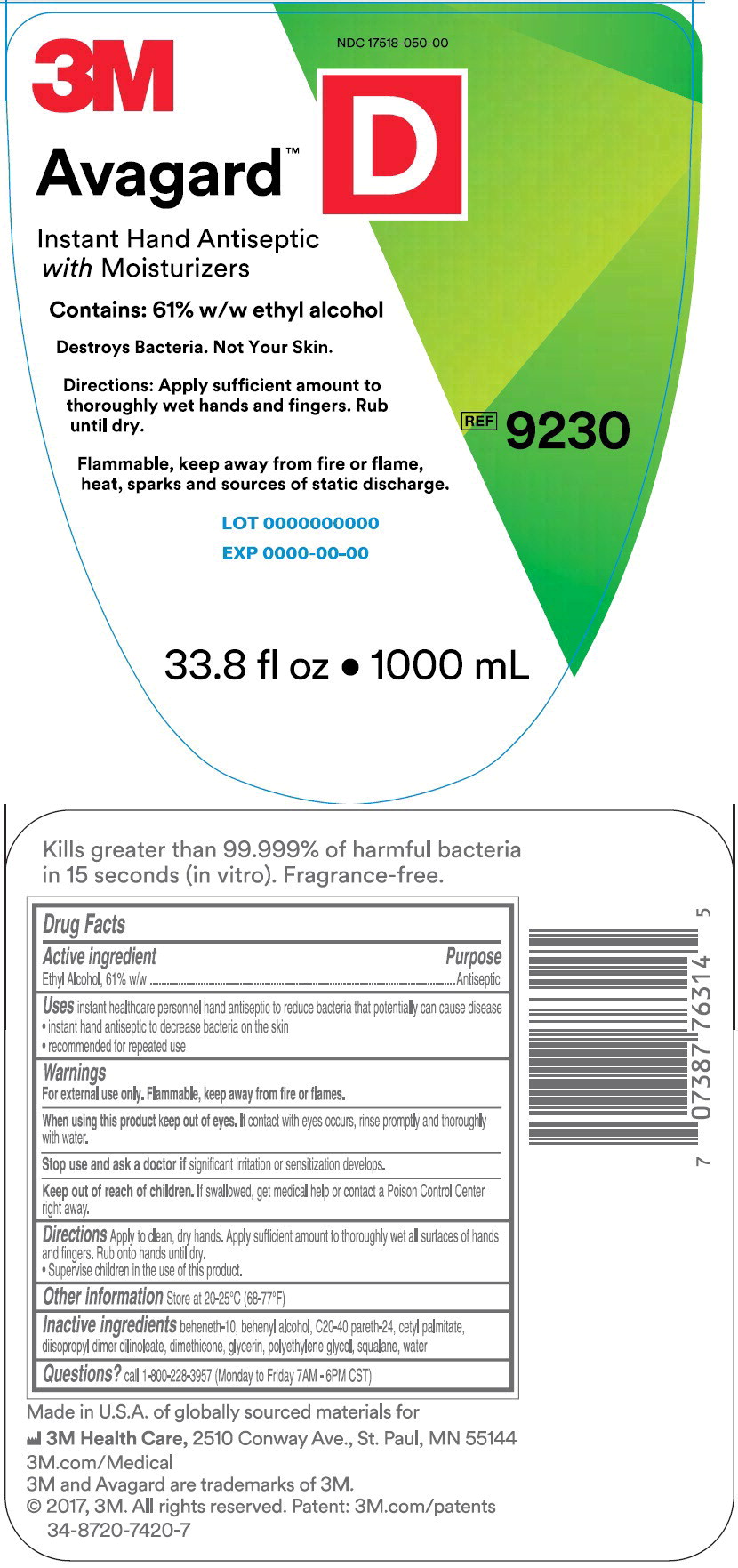
Uses
- instant healthcare personnel hand antiseptic to reduce bacteria that potentially can cause disease
- instant hand antiseptic to decrease bacteria on the skin
- recommended for repeated use
Warnings
For external use only. Flammable, keep away from fire or flames.
Directions
Apply to clean, dry hands. Apply sufficient amount to thoroughly wet all surfaces of hand and fingers. Rub on to hands until dry.
- Supervise children in the use of this product.
Inactive ingredients
beheneth-10, behenyl alcohol, C20-40 pareth-24, cetyl palmitate, diisopropyl dimer dilinoleate, dimethicone, glycerin, polyethylene glycol, squalane, water
Questions?
call 1-800-228-3957 (Monday to Friday 7AM-6PM CST)
Made in U.S.A. of globally sourced materials for
3M Health Care, 2510 Conway Ave., St. Paul, MN 55144
3M.com/Medical
3M and Avagard are trademarks of 3M.
© 2017, 3M. All rights reserved. Patent: 3M.com/patents
34-8720-7420-7
Principle Display Panel – 1000 mL Bottle Label
NDC 17518-050-00
3M
Avagard™ D
Instant Hand Antiseptic with Moisturizer
Contains: 61% w/w ethyl alcohol
Destroys Bacteria. Not Your Skin.
Directions: Apply sufficient amount to thoroughly wet hands and fingers.
Rub until dry.
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge
REF 9230
33.8 fl oz • 1000 mL