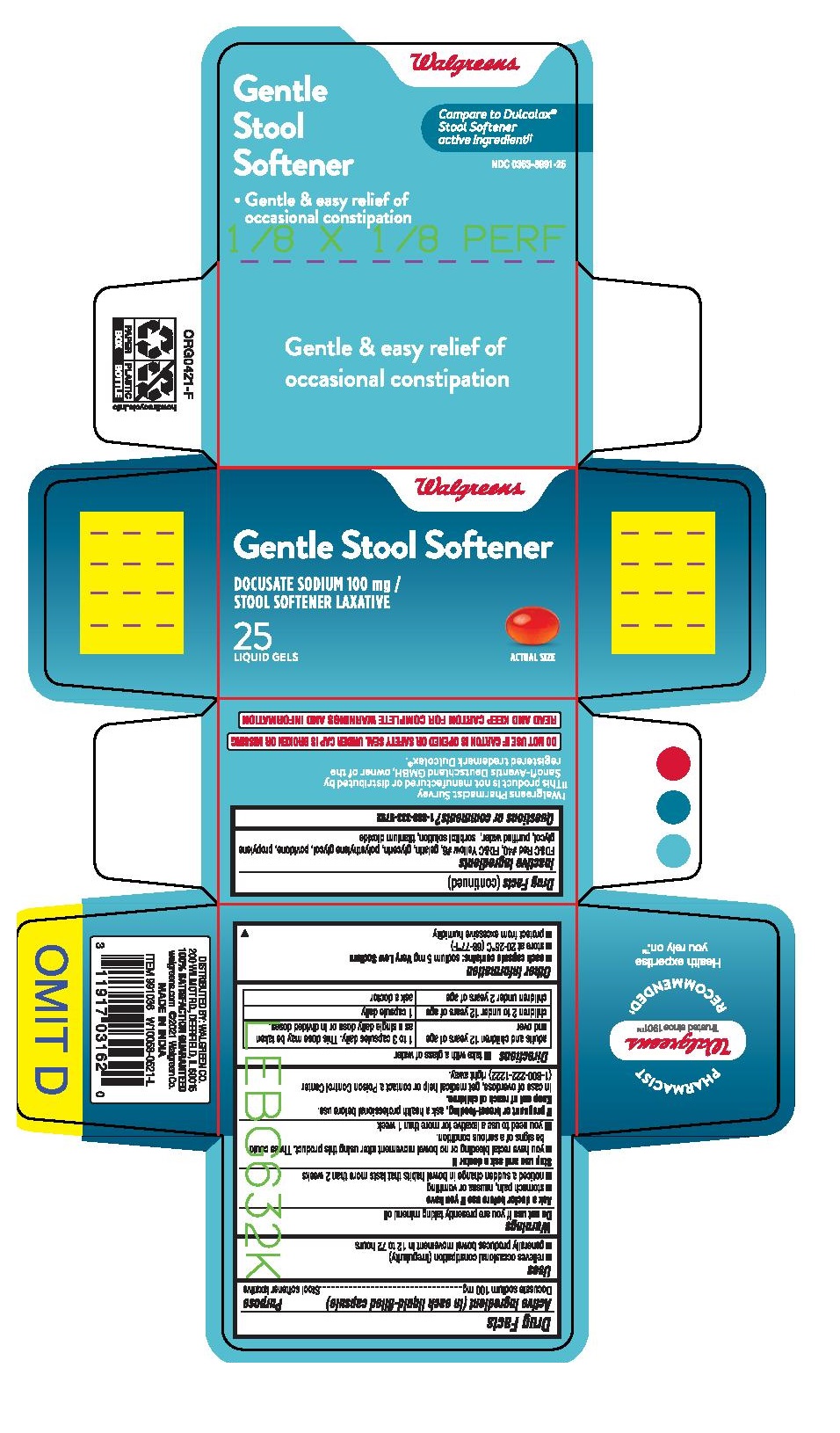
Uses
- relieves of occasional constipation (irregularity)
- this product generally produces a bowel movement within 12 to 72 hours
Ask a doctor before use if you have
- stomach pain, nausea or vomittiong
- a sudden change in bowel habits that lasts more than 2 weeks
Stop use and ask a doctor if you
- you have rectal bleeding ot no bowel movement after using this product. These could be signs of a serious condition.
- yo need to use laxative for more than 1 week
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.1(800)222-1222
Directions
take with a glass of water
take by mouth. Doses may be taken as a single daily dose or in divided doses.
|
Adults and children 12 years and over |
take 1 to 3 capsules daily. |
|
Children 2 to under 12 years of age |
1 capsule daily. |
|
Children under 2 years of age |
Ask a doctor |
Other information
- Store at room temperature 20-25°C (68-77°F)
- protect from excessive humidity
- do not use this product of the safety seal under the cap is torn or missing