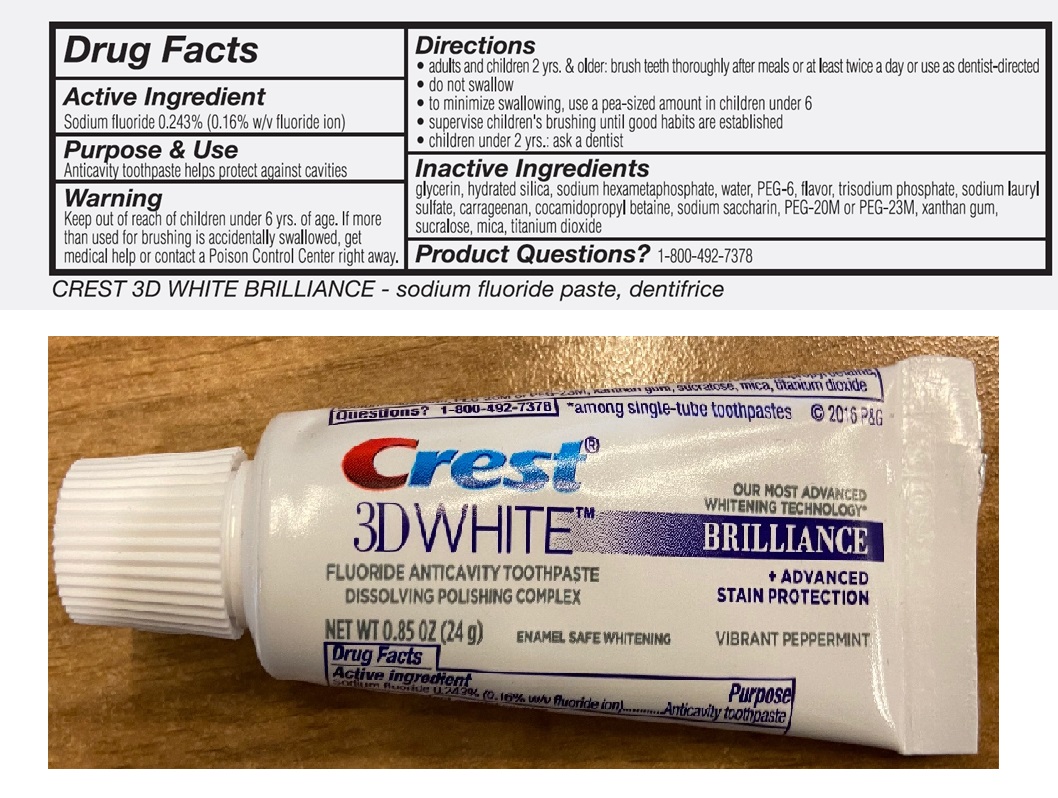
Active Ingredient
Sodium fluoride 0.243% (0.16% w/v fluoride ion)
Use
toothpate helps protect against cavities
Warning
Keep out of reach of children
under 6 yrs. of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as dentist-directed
- do not swallow
- to minimize swallowing, use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs: ask a dentist
Inactive Ingredients
glycerin, hydrated silica, sodium hexametaphosphate, water, PEG-6, flavor, trisodium phosphate, sodium lauryl sulfate, carrageenan, cocamidopropyl betaine, sodium saccharin, PEG-20M, xanthan gum, sucralose, mica, titanium dioxide
Product Questions?
1-800-492-7378
Package Labeling: Kit

Package Labeling:24g