12 HOUR ALLERGY D- cetirizine hcl, pseudoephedrine hcl tablet, extended release
Meijer Distribution Inc
----------
Meijer Distribution, Inc. 12 Hour Allergy-D Drug Facts
Uses
- •
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itching of the nose or throat
- •
- nasal congestion
- •
- reduces swelling of nasal passages
- •
- temporarily relieves sinus congestion and pressure
- •
- temporarily restores freer breathing through the nose
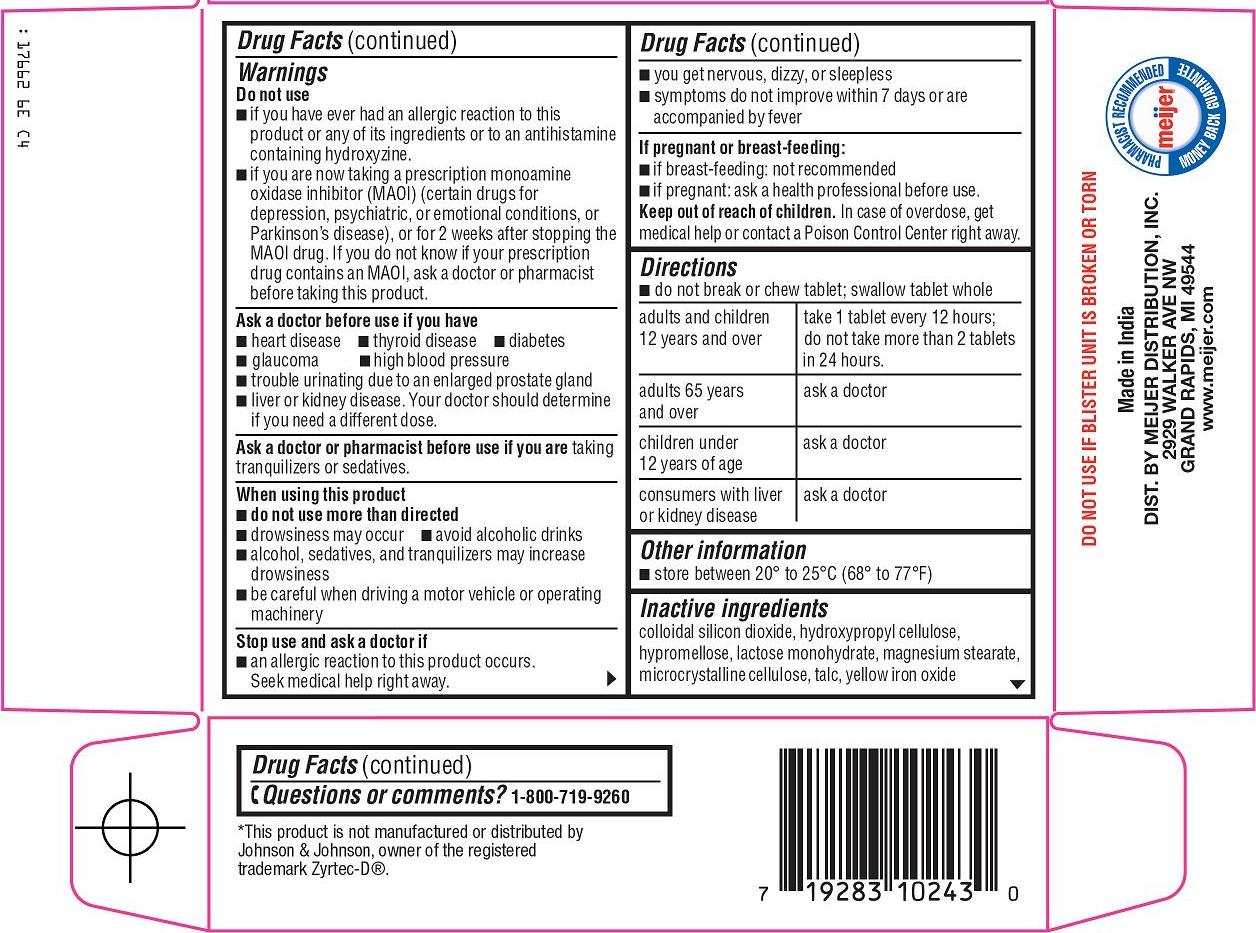
Warnings
Do not use
- •
- if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- heart disease
- •
- thyroid disease
- •
- diabetes
- •
- glaucoma
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
- •
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- •
- do not use more than directed
- •
- drowsiness may occur
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- •
- an allergic reaction to this product occurs. Seek medical help right away.
- •
- you get nervous, dizzy, or sleepless
- •
- symptoms do not improve within 7 days or are accompanied by fever
Directions
- •
- do not break or chew tablet; swallow tablet whole
|
adults and children 12 years and over |
take 1 tablet every 12 hours; do not take more than 2 tablets in 24 hours. |
|
adults 65 years and over |
ask a doctor |
|
children under 12 years of age |
ask a doctor |
|
consumers with liver or kidney disease |
ask a doctor |
Inactive ingredients
colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, talc, yellow iron oxide
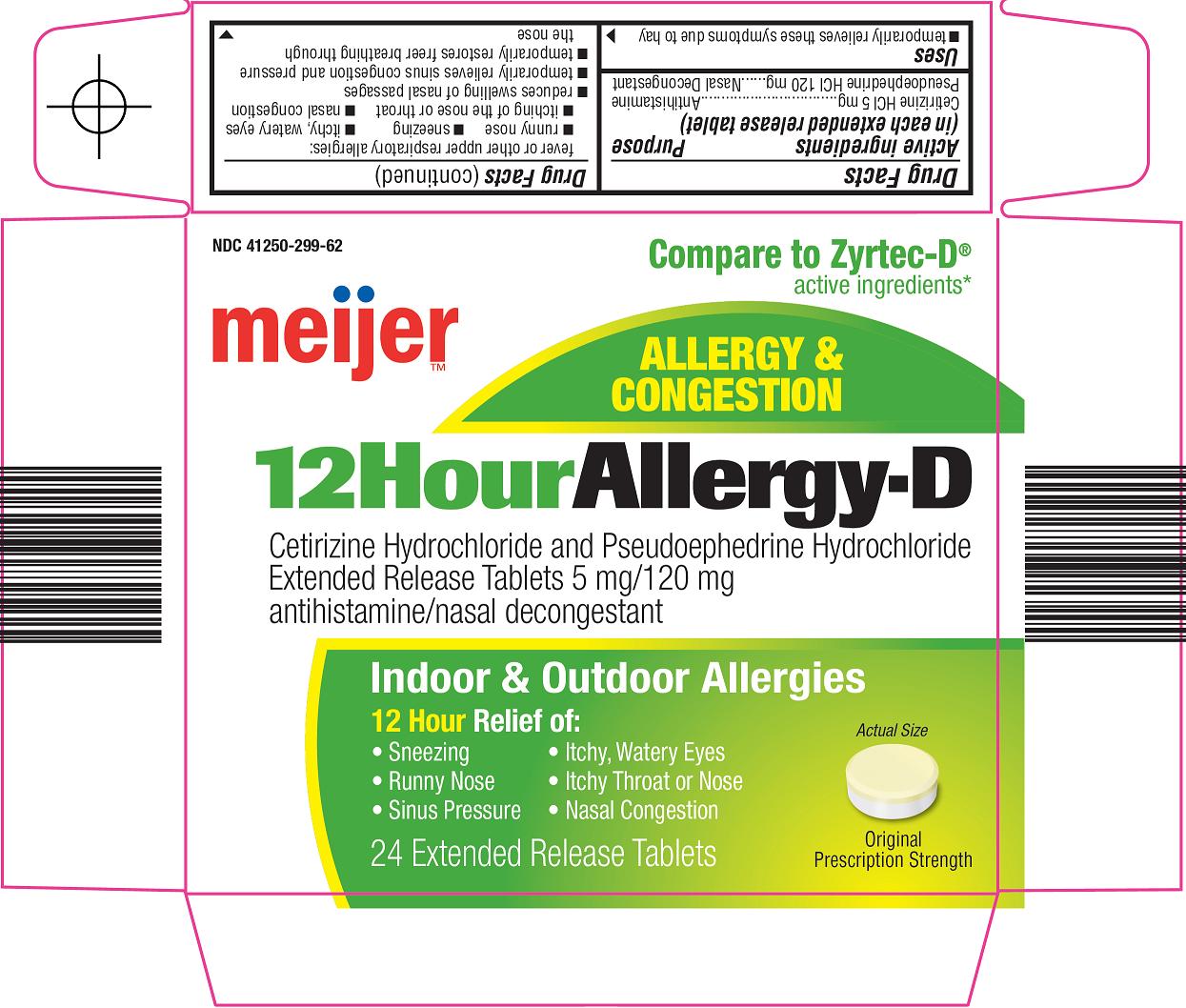
Principal Display Panel
Compare to Zyrtec-D® active ingredients
ALLERGY & CONGESTION
12 Hour Allergy-D
Cetirizine Hydrochloride and Pseudoephedrine Hyrdochloride
Extended Release Tablets 5 mg/120 mg
antihistamine/nasal decongestant
Indoor & Outdoor Allergies
12 Hour Relief of:
Sneezing
Itchy, Watery Eyes
Runny Nose
Itchy Throat or Nose
Sinus Pressure
Nasal Congestion
Actual Size
Original Prescription Strength
| 12 HOUR ALLERGY D
cetirizine hcl, pseudoephedrine hcl tablet, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Meijer Distribution Inc (006959555) |