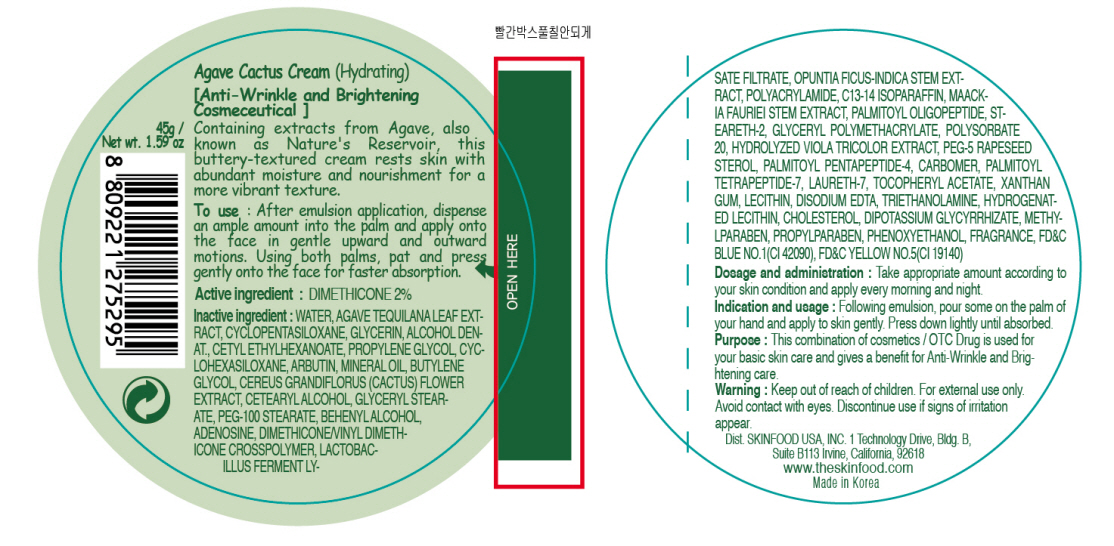
Inactive ingredients:
WATER, AGAVE TEQUILANA LEAF EXTRACT, CYCLOPENTASILOXANE, GLYCERIN, ALCOHOL DENAT., CETYL ETHYLHEXANOATE, CYCLOHEXASILOXANE, PROPYLENE GLYCOL, ARBUTIN, MINERAL OIL, BUTYLENE GLYCOL, CEREUS GRANDIFLORUS (CACTUS) FLOWER EXTRACT, CETEARYL ALCOHOL, GLYCERYL STEARATE, PEG-100 STEARATE, BEHENYL ALCOHOL, ADENOSINE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, LACTOBACILLUS FERMENT LYSATE FILTRATE, OPUNTIA FICUS-INDICA STEM EXTRACT, POLYACRYLAMIDE, C13-14 ISOPARAFFIN, MAACKIA FAURIEI STEM EXTRACT, PALMITOYL OLIGOPEPTIDE, STEARETH-2, GLYCERYL POLYMETHACRYLATE, POLYSORBATE 20, HYDROLYZED VIOLA TRICOLOR EXTRACT, PEG-5 RAPESEED STEROL, PALMITOYL PENTAPEPTIDE-4, CARBOMER, PALMITOYL TETRAPEPTIDE-7, LAURETH-7, TOCOPHERYL ACETATE, XANTHAN GUM, LECITHIN, DISODIUM EDTA, TRIETHANOLAMINE, HYDROGENATED LECITHIN, CHOLESTEROL, DIPOTASSIUM GLYCYRRHIZATE, METHYLPARABEN, PROPYLPARABEN, PHENOXYETHANOL, FRAGRANCE
Purpose:
This combination of cosmetics is used for your basic skin care and gives a benefit for Anti-Wrinkle and Brightening care.
Warnings:
For external use only. Avoid contact with eyes. Discontinue use if signs of irritation appear.
Indication and usage:
Following emulsion, pour some on the palm of your hand and apply to skin gently. Press down lightly until absorbed.