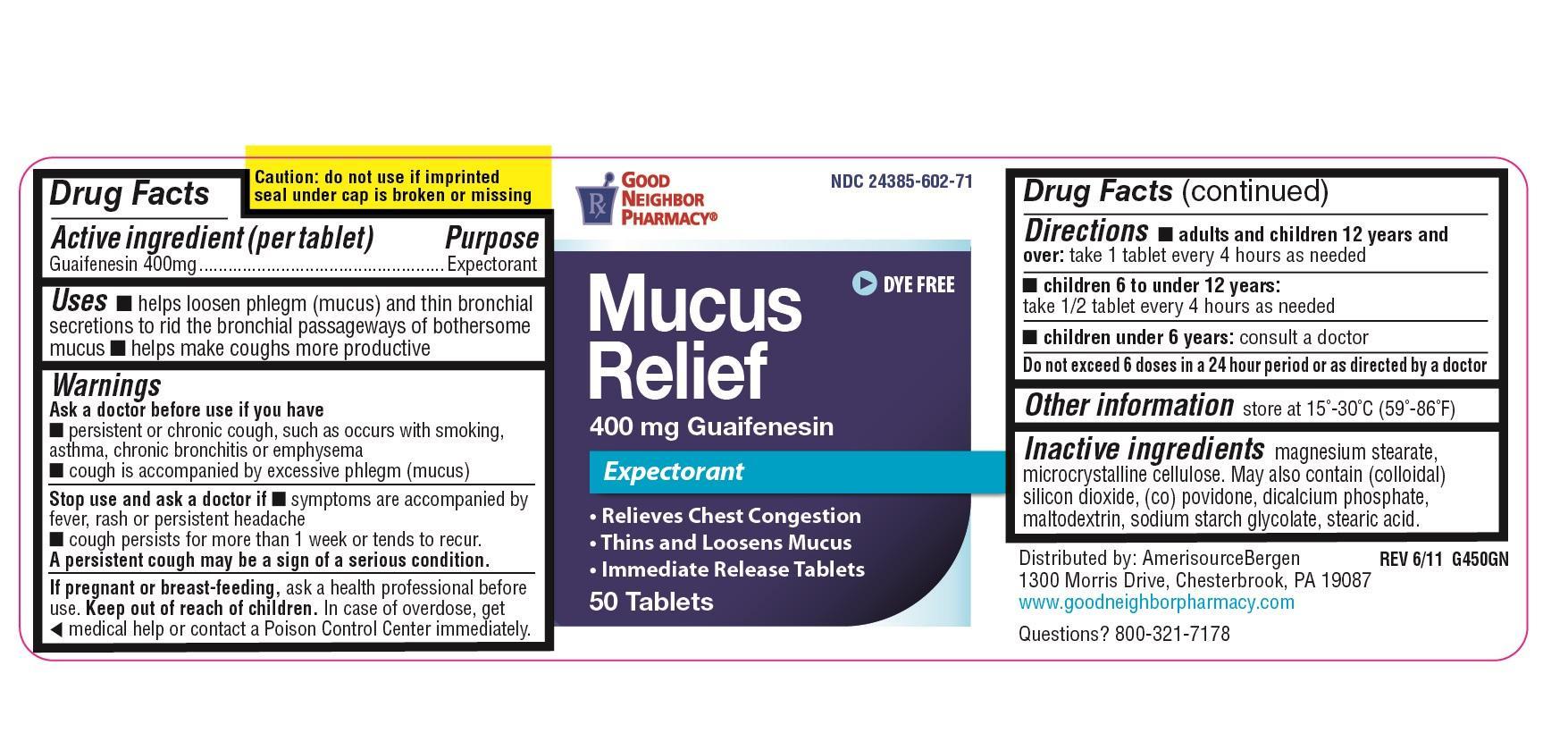
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus
- helps make coughs more productive
Warnings
Ask doctor before use if you have
- persistent or chronic cough, such as occurs with smoking, asthma, bronchitis or emphysema
- cough is accompanied by excessive phlegm (mucus)
Stop use and ask doctor if
- Symptoms are accompanied by fever, rash or persistent headache
- cough persists for more than 1 week or tends to recur
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control
Center immediately.
Directions
- Adults and children 12 years of age and over: take 1 tablet every 4 hours as needed
- Children 6 to 10 under 12 years of age: take 1/2 tablet every 4 hours as needed
- Children under 6 years of age: consult a doctor