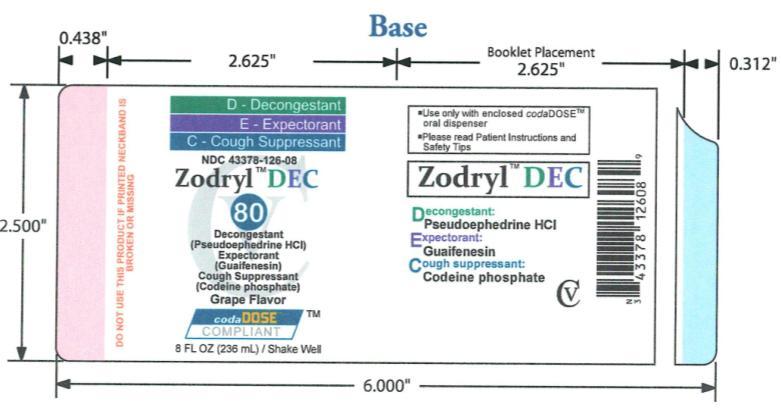
OTC - ACTIVE INGREDIENT
Codeine phosphate 1 mg/1mL: cough suppressant; Guaifenesin 20 mg/1mL: expectorant; Pseudoephedrine hydrochloride 3 mg/1mL: decongestant
PURPOSE
Temporarily relieves: cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants; the intensity of coughing; the impulse to cough to help you go to sleep; temporarily relieves nasal congestion due to a cold; temporarily restores freer breathing through the nose; helps loosen phlegm (mucus) and thin bronchial passageways of bothersome mucus and makes coughs more productive
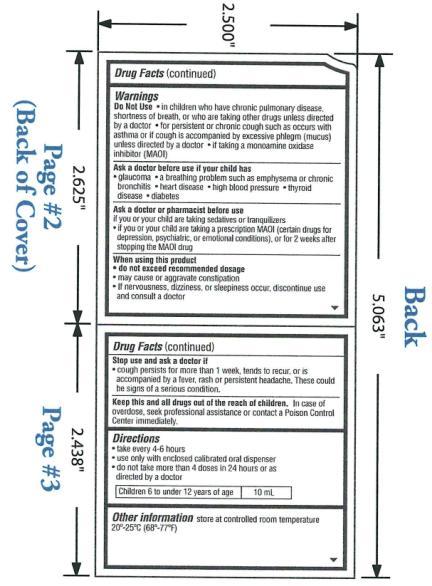
Warnings
OTC - DO NOT USE
in children who have chronic pulmonary disease, shortness of breath, or such as occurs with asthma or if cough is accompanied by excessive phlegm (mucus) unless directed by a doctor; if taking a monoamine oxidase inhibitor (MAOI)
OTC - ASK DOCTOR
if your child has glaucoma, a breathing problem such as emphysema or chronic bronchitis, heart disease, high blood pressure, thyroid disease, diabetes.
OTC - ASK DOCTOR/PHARMACIST SECTION
if you or your child are taking sedatives or tranquilizers; if you or your child are taking prescription MAOI (certain drugs for depression, psychiatric, or emotional conditions), or for 2 weeks after stopping the MAOI drug.
OTC - WHEN USING THIS PRODUCT
do not exceed recommended dosage; may cause or aggravate constipation; if nervousness, dizziness, or sleepiness occur, discontinue use and consult a doctor
OTC - STOP USE AND ASK A DOCTOR IF
cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache. These could be signs of a serious condition.
OTC - KEEP THESE AND ALL DRUGS OUT OF REACH OF CHILDREN
In case of overdose, seek professional assistance for contact a Poison Control Center immediately.
Directions:
-
Take every 4-6 hours
-
Use only with enclosed calibrated oral dispenser
-
Do not take more than 4 doses in 24 hours or as directed by a doctor
Children 6 to under 12 years of age: 10mL
Other information store at controlled room temperature 20°-25°C (68°-77°F).
INACTIVE INGREDIENT
Bittermask, citric acid, FD& C blue #1, FD& C red #40, galloquinate, glycerin, grape flavor, magnesium aluminometasilicate, methylparaben, purified water, sodium citrate dihydrate, sucralose, xanthan gum