HEAD AND SHOULDERS DUAL SACHETS CLASSIC CLEAN- pyrithione zinc
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
head &
shoulders
classic clean
pyrithione zinc dandruff shampoo
Drug Facts
Active ingredient
Pyrithione zinc 1%
Uses
helps prevent recurrence of flaking and itching associated with dandruff.
Warnings
For external use only.
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
- condition worsens or does not improve after regular use of this product as directed.
Keep this and all drugs out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- for best results use
at least twice a week or as directed by a doctor.
- for maximum dandruff control, use every time you shampoo.
- shake before use.
- wet hair, massage onto scalp, rinse, repeat if desired.
Inactive ingredients
Water, sodium lauryl sulfate, sodium laureth sulfate, glycol distearate, zinc carbonate, sodium chloride, sodium xylenesulfonate, cocamidopropyl betaine, fragrance, dimethicone, sodium benzoate, guar hydroxypropyltrimonium chloride, magnesium carbonate hydroxide, methylchloroisothiazolinone, methylisothiazolinone, blue 1, red 33.
Questions (or comments)?
1-800-723-9569
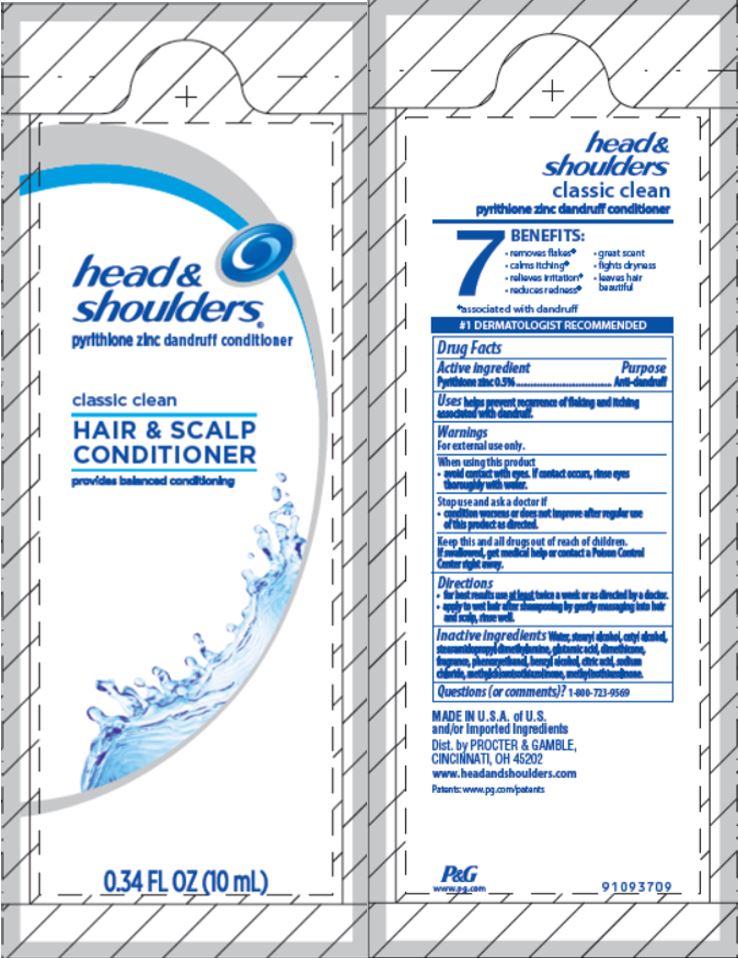
head &
shoulders
classic clean
pyrithione zinc dandruff conditioner
Drug Facts
Active ingredient
Pyrithione zinc 0.5%
Uses
helps prevent recurrence of flaking and itching associated with dandruff.
Warnings
For external use only.
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
- condition worsens or does not improve after regular use of this product as directed.
Keep this and all drugs out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- for best results use
at least twice a week or as directed by a doctor.
- apply to wet hair after shampooing by gently massaging into hair and scalp, rinse well.
Inactive ingredients
Water, stearyl alcohol, cetyl alcohol, stearamidopropyl dimethylamine, glutamic acid, dimethicone, fragrance, phenoxyethanol, benzyl alcohol, citric acid, sodium chloride, methylchloroisothiazolinone, methylisothiazolinone.
Questions (or comments)?
1-800-723-9569
Dist. by PROCTER & GAMBLE,
CINCINNATI, OH 45202
PRINCIPAL DISPLAY PANEL - 10 mL Pouch Label - Shampoo
head &
shoulders
®
pyrithione zinc
dandruff shampoo
classic clean
america's #1 dandruff shampoo**
FLAKE FREE,*
UP TO
100%
GURANTEED^
0.34 FL OZ (10 mL)

PRINCIPAL DISPLAY PANEL - 10 mL Pouch Label - Conditioner
head &
shoulders
®
pyrithione zinc
dandruff conditioner
classic clean
HAIR & SCALP
CONDITIONER
provides balanced conditioning
0.34 FL OZ (10 mL)