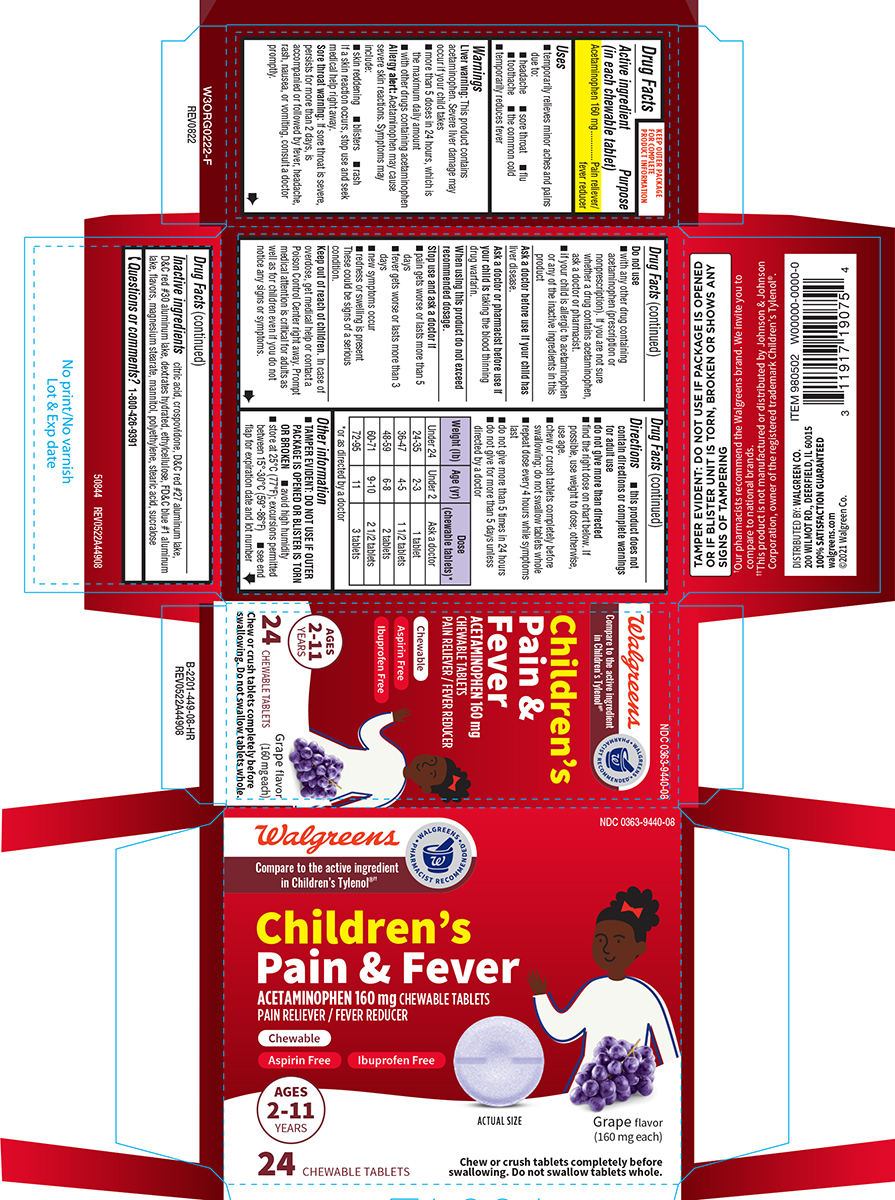
Uses
- temporarily relieves minor aches and pains due to:
- headache
- sore throat
- flu
- toothache
- the common cold
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
| Weight (lb) | Age (yr) | Dose (chewable tablets)*
|
| Under 24 | Under 2 | Ask a doctor |
| 24-35 | 2-3 | 1 tablet |
| 36-47 | 4-5 | 1 1/2 tablets |
| 48-59 | 6-8 | 2 tablets |
| 60-71 | 9-10 | 2 1/2 tablets |
| 72-95 | 11 | 3 tablets |
*or as directed by a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- avoid high humidity
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
citric acid, crospovidone, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates hydrated, ethylcellulose, FD&C blue #1 aluminum lake, flavors, magnesium stearate, mannitol, polyethylene, stearic acid, sucralose
Principal display panel
NDC 0363-9440-08
Walgreens
Compare to the active ingredient
in Children’s Tylenol®††
• WALGREENS •
PHARMACIST RECOMMENDED†
Children’s
Pain & Fever
ACETAMINOPHEN 160 mg CHEWABLE TABLETS
PAIN RELIEVER / FEVER REDUCER
Chewable
Aspirin Free
Ibuprofen Free
AGES
2-11
YEARS
24 CHEWABLE TABLETS
ACTUAL SIZE Grape flavor (160 mg each)
Chew or crush tablets completely before
swallowing. Do not swallow tablets whole.
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED
OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY
SIGNS OF TAMPERING
Walgreens Pharmacist Recommended
Walgreens Pharmacist Survey
†Our pharmacists recommend the Walgreens brand. We invite you to
compare to national brands.
††This product is not manufactured or distributed by Johnson & Johnson
Corporation, owner of the registered trademark Children’s Tylenol®.
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
walgreens.com
©2021 Walgreen Co.
50844 REV0522A44908
Walgreens 44-449